Retinal regeneration in the Xenopus laevis tadpole: a new model system
- PMID: 19461929
- PMCID: PMC2684558
Retinal regeneration in the Xenopus laevis tadpole: a new model system
Abstract
Purpose: Retinal regeneration research holds potential for providing new avenues for the treatment of degenerative diseases of the retina. Various animal models have been used to study retinal regeneration over the years, providing insights into different aspects of this process. However the mechanisms that drive this important phenomenon remain to be fully elucidated. In the present study, we introduce and characterize a new model system for retinal regeneration research that uses the tadpole of the African clawed frog, Xenopus laevis.
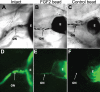
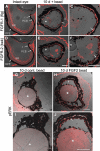
Methods: The neural retina was surgically removed from Xenopus laevis tadpoles at stages 51-54, and a heparin-coated bead soaked in fibroblast growth factor 2 (FGF-2) was introduced in the eyes to induce regeneration. Histological and immunohistochemical analyses as well as DiI tracing were performed to characterize the regenerate. A similar surgical approach but with concomitant removal of the anterior portion of the eye was used to assess the capacity of the retinal pigmented epithelium (RPE) to regenerate a retina. Immunohistochemistry for FGF receptors 1 and 2 and phosphorylated extracellular signal-regulated protein kinase (pERK) was performed to start elucidating the intracellular mechanisms involved in this process. The role of the mitogen activated protein kinase (MAPK) pathway was confirmed through a pharmacological approach using the MAPK kinase (MEK) inhibitor U0126.
Results: We observed that Xenopus laevis tadpoles were able to regenerate a neural retina upon induction with FGF-2 in vivo. The regenerated tissue has the characteristics of a differentiated retina, as assessed by the presence and distribution of different retinal cell markers, and DiI tracing indicated that it is able to form an optic nerve. We also showed that retinal regeneration in this system could take place independently of the presence of the anterior eye tissues. Finally, we demonstrated that FGF-2 treatment induces ERK phosphorylation in the pigmented epithelia 10 days after retinectomy, and that inhibition of the MAPK pathway significantly decreases the amount of retina regenerated at 30 days post-operation.
Conclusions: Regeneration of a complete neural retina can be achieved in larval Xenopus laevis through activation of the MAPK signaling pathway by administering exogenous FGF-2. This mechanism is conserved in other animal models, which can regenerate their retina via pigmented epithelium transdifferentiation. Our results provide an alternative approach to retinal regeneration studies, capitalizing on the advantages of the Xenopus laevis tadpole as a model system.
Figures




Similar articles
-
Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: transdifferentiation of retinal pigmented epithelium regenerates the neural retina.Dev Biol. 2007 Mar 1;303(1):45-56. doi: 10.1016/j.ydbio.2006.11.024. Epub 2006 Nov 21. Dev Biol. 2007. PMID: 17184765
-
Retina regeneration in the chick embryo is not induced by spontaneous Mitf downregulation but requires FGF/FGFR/MEK/Erk dependent upregulation of Pax6.Mol Vis. 2007 Jan 24;13:57-65. Mol Vis. 2007. PMID: 17277739 Free PMC article.
-
Retinal stem/progenitor cells in the ciliary marginal zone complete retinal regeneration: a study of retinal regeneration in a novel animal model.Dev Neurobiol. 2014 Jul;74(7):739-56. doi: 10.1002/dneu.22169. Epub 2014 Feb 18. Dev Neurobiol. 2014. PMID: 24488715
-
The African clawed frog Xenopus laevis: A model organism to study regeneration of the central nervous system.Neurosci Lett. 2017 Jun 23;652:82-93. doi: 10.1016/j.neulet.2016.09.054. Epub 2016 Sep 29. Neurosci Lett. 2017. PMID: 27693567 Review.
-
Transdifferentiation of ocular tissues in larval Xenopus laevis.Differentiation. 1988 Nov;39(1):4-15. doi: 10.1111/j.1432-0436.1988.tb00074.x. Differentiation. 1988. PMID: 3073094 Review.
Cited by
-
Induction of ectopic retina-like tissue by transgenic expression of neurogenin.PLoS One. 2015 Jan 30;10(1):e0116171. doi: 10.1371/journal.pone.0116171. eCollection 2015. PLoS One. 2015. PMID: 25635399 Free PMC article.
-
Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina.Nat Rev Neurosci. 2010 Aug;11(8):563-76. doi: 10.1038/nrn2880. Nat Rev Neurosci. 2010. PMID: 20648062 Free PMC article. Review.
-
Preliminary study on electrophysiological changes after cellular autograft in age-related macular degeneration.Medicine (Baltimore). 2014 Dec;93(29):e355. doi: 10.1097/MD.0000000000000355. Medicine (Baltimore). 2014. PMID: 25546695 Free PMC article.
-
Transducing bioelectric signals into epigenetic pathways during tadpole tail regeneration.Anat Rec (Hoboken). 2012 Oct;295(10):1541-51. doi: 10.1002/ar.22495. Epub 2012 Aug 29. Anat Rec (Hoboken). 2012. PMID: 22933452 Free PMC article. Review.
-
MAPK/ERK Pathway as a Central Regulator in Vertebrate Organ Regeneration.Int J Mol Sci. 2022 Jan 27;23(3):1464. doi: 10.3390/ijms23031464. Int J Mol Sci. 2022. PMID: 35163418 Free PMC article. Review.
References
-
- Reh TA, Pittack C. Transdifferentiation and retinal regeneration. Semin Cell Biol. 1995;6:137–42. - PubMed
-
- Mitashov VI. Mechanisms of retina regeneration in urodeles. Int J Dev Biol. 1996;40:833–44. - PubMed
-
- Mitashov VI. Retinal regeneration in amphibians. Int J Dev Biol. 1997;41:893–905. - PubMed
-
- Tsonis PA. Regeneration in vertebrates. Dev Biol. 2000;221:273–84. - PubMed
-
- Haynes T, Del Rio-Tsonis K. Retina repair, stem cells and beyond. Curr Neurovasc Res. 2004;1:231–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
