Recrudescent Plasmodium berghei from pregnant mice displays enhanced binding to the placenta and induces protection in multigravida
- PMID: 19461965
- PMCID: PMC2680968
- DOI: 10.1371/journal.pone.0005630
Recrudescent Plasmodium berghei from pregnant mice displays enhanced binding to the placenta and induces protection in multigravida
Abstract
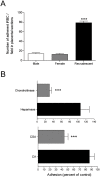
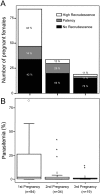
Pregnancy-associated malaria (PAM) is associated with placenta pathology and poor pregnancy outcome but the mechanisms that control the malaria parasite expansion in pregnancy are still poorly understood and not amenable for study in human subjects. Here, we used a set of new tools to re-visit an experimental mouse model of pregnancy-induced malaria recrudescence, BALB/c with chronic Plasmodium berghei infection. During pregnancy 60% of the pre-exposed primiparous females showed pregnancy-induced malaria recrudescence and we demonstrated that the recrudescent P. berghei show an unexpected enhancement of the adherence to placenta tissue sections with a marked specificity for CSA. Furthermore, we showed that the intensity of parasitemia in primigravida was quantitatively correlated with the degree of thickening of the placental tissue and up-regulation of inflammation-related genes such as IL10. We also confirmed that the incidence of pregnancy-induced recrudescence, the intensity of the parasitemia peak and the impact on the pregnancy outcome decreased gradually from the first to the third pregnancy. Interestingly, placenta pathology and fetal impairment were also observed at low frequency among non-recrudescent females. Together, the data raise the hypothesis that recrudescent P. berghei displays selected specificity for the placenta tissue enabling on one hand, the triggering of the pathological process underlying PAM and on the other hand, the induction of PAM protection mechanisms that are revealed in subsequent pregnancies. Thus, by exploiting P. berghei pregnancy-induced recrudescence, this experimental system offers a mouse model to study the susceptibility to PAM and the mechanisms of disease protection in multigravida.
Conflict of interest statement
Figures





Similar articles
-
Distinct placental malaria pathology caused by different Plasmodium berghei lines that fail to induce cerebral malaria in the C57BL/6 mouse.Malar J. 2012 Jul 16;11:231. doi: 10.1186/1475-2875-11-231. Malar J. 2012. PMID: 22799533 Free PMC article.
-
Murine Model for Preclinical Studies of Var2CSA-Mediated Pathology Associated with Malaria in Pregnancy.Infect Immun. 2016 May 24;84(6):1761-1774. doi: 10.1128/IAI.01207-15. Print 2016 Jun. Infect Immun. 2016. PMID: 27045035 Free PMC article.
-
Cytokine response to pregnancy-associated recrudescence of Plasmodium berghei infection in mice with pre-existing immunity to malaria.Malar J. 2013 Nov 1;12:387. doi: 10.1186/1475-2875-12-387. Malar J. 2013. PMID: 24180253 Free PMC article.
-
Cytoadhesion of Plasmodium falciparum-infected erythrocytes and the infected placenta: a two-way pathway.Braz J Med Biol Res. 2006 Dec;39(12):1525-36. doi: 10.1590/s0100-879x2006001200003. Braz J Med Biol Res. 2006. PMID: 17160261 Review.
-
The epidemiology and consequences of maternal malaria: a review of immunological basis.Acta Trop. 2003 Jul;87(2):193-205. doi: 10.1016/s0001-706x(03)00097-4. Acta Trop. 2003. PMID: 12826295 Review.
Cited by
-
Development of severe pathology in immunized pregnant mice challenged with lethal malaria parasites.Infect Immun. 2013 Oct;81(10):3865-71. doi: 10.1128/IAI.00749-13. Epub 2013 Jul 29. Infect Immun. 2013. PMID: 23897619 Free PMC article.
-
TLR4-Mediated Placental Pathology and Pregnancy Outcome in Experimental Malaria.Sci Rep. 2017 Aug 17;7(1):8623. doi: 10.1038/s41598-017-08299-x. Sci Rep. 2017. PMID: 28819109 Free PMC article.
-
IFNGR1 signaling is associated with adverse pregnancy outcomes during infection with malaria parasites.PLoS One. 2017 Nov 8;12(11):e0185392. doi: 10.1371/journal.pone.0185392. eCollection 2017. PLoS One. 2017. PMID: 29117241 Free PMC article.
-
Contribution of Murine Models to the Study of Malaria During Pregnancy.Front Microbiol. 2019 Jun 19;10:1369. doi: 10.3389/fmicb.2019.01369. eCollection 2019. Front Microbiol. 2019. PMID: 31275284 Free PMC article. Review.
-
In vivo multiphoton microscopy technique to reveal the physiology of the mouse placenta.Am J Reprod Immunol. 2012 Sep;68(3):271-8. doi: 10.1111/j.1600-0897.2012.01161.x. Epub 2012 May 24. Am J Reprod Immunol. 2012. PMID: 22626451 Free PMC article.
References
-
- WHO. A strategic framework for malaria prevention and control during pregnancy in the African region. Geneva: World Health Organization AFR/MAL; 2004.
-
- Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, et al. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55:33–41. - PubMed
-
- Shulman CE, Marshall T, Dorman EK, Bulmer JN, Cutts F, et al. Malaria in pregnancy: adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Trop Med Int Health. 2001;6:770–778. - PubMed
-
- Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–1745. Epub 2000 May 1715. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

