Mechanistic basis for differential inhibition of the F1Fo-ATPase by aurovertin
- PMID: 19462418
- PMCID: PMC2757082
- DOI: 10.1002/bip.21262
Mechanistic basis for differential inhibition of the F1Fo-ATPase by aurovertin
Abstract
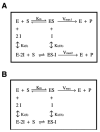
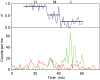
The mitochondrial F(1)F(o)-ATPase performs the terminal step of oxidative phosphorylation. Small molecules that modulate this enzyme have been invaluable in helping decipher F(1)F(o)-ATPase structure, function, and mechanism. Aurovertin is an antibiotic that binds to the beta subunits in the F(1) domain and inhibits F(1)F(o)-ATPase-catalyzed ATP synthesis in preference to ATP hydrolysis. Despite extensive study and the existence of crystallographic data, the molecular basis of the differential inhibition and kinetic mechanism of inhibition of ATP synthesis by aurovertin has not been resolved. To address these questions, we conducted a series of experiments in both bovine heart mitochondria and E. coli membrane F(1)F(o)-ATPase. Aurovertin is a mixed, noncompetitive inhibitor of both ATP hydrolysis and synthesis with lower K(i) values for synthesis. At low substrate concentrations, inhibition is cooperative suggesting a stoichiometry of two aurovertin per F(1)F(o)-ATPase. Furthermore, aurovertin does not completely inhibit the ATP hydrolytic activity at saturating concentrations. Single-molecule experiments provide evidence that the residual rate of ATP hydrolysis seen in the presence of saturating concentrations of aurovertin results from a decrease in the binding change mechanism by hindering catalytic site interactions. The results from these studies should further the understanding of how the F(1)F(o)-ATPase catalyzes ATP synthesis and hydrolysis.
(c) 2009 Wiley Periodicals, Inc. Biopolymers 91: 830-840, 2009.
Figures

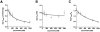





References
-
- Linnett PE, Beechey RB. Methods Enzymol. 1979;55:472–518. - PubMed
-
- Lardy HA, Connelly JL, Johnson D. Biochemistry. 1964;3:1961–1968. - PubMed
-
- Verschoor GJ, van der Sluis PR, Slater EC. Biochim Biophys Acta. 1977;462:438–449. - PubMed
-
- Douglas MG, Koh Y, Dockter ME, Schatz G. J Biol Chem. 1977;252:8333–8335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

