Update of cylindromatosis gene (CYLD) mutations in Brooke-Spiegler syndrome: novel insights into the role of deubiquitination in cell signaling
- PMID: 19462465
- PMCID: PMC3243308
- DOI: 10.1002/humu.21024
Update of cylindromatosis gene (CYLD) mutations in Brooke-Spiegler syndrome: novel insights into the role of deubiquitination in cell signaling
Abstract
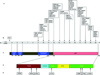
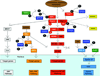
Germline mutations in the cylindromatosis (CYLD) gene have been described in families with cylindromas, trichoepitheliomas, and/or spiradenomas. Brooke-Spiegler syndrome (BSS) is the autosomal dominant predisposition to skin appendageal neoplasms including cylindromas, trichoepitheliomas, and/or spiradenomas. We review the clinical features, molecular genetics, and the animal models of BSS. To date, a total of 51 germline CYLD mutations have been reported, occurring in exons 9-20, in 73 families with diverse ethnic and racial backgrounds. Of 51 mutations, 86% are expected to lead to truncated proteins. The seven missense mutations reported to date occur only within the ubiquitin (Ub)-specific protease (USP) domain of the CYLD protein and most are associated exclusively with multiple familial trichoepithelioma (MFT). CYLD functions as a tumor suppressor gene. CYLD encodes a deubiquitinating (DUB) enzyme that negatively regulates the nuclear factor (NF)-kappaB and c-Jun N-terminal kinase (JNK) pathways. CYLD DUB activity is highly specific for lysine 63 (K63)-linked Ub chains but has been shown to act on K48-linked Ub chains as well. In 2008, the CYLD USP domain was crystallized, revealing that the truncated Fingers subdomain confers CYLD's unique specificity for K63-linked Ub chains. Recent work using animal models revealed new roles for CYLD in immunity, lipid metabolism, spermatogenesis, osteoclastogenesis, antimicrobial defense, and inflammation.
Figures
References
-
- Almeida S, Maillard C, Itin P, Hohl D, Huber M. Five new CYLD mutations in skin appendage tumors and evidence that aspartic acid 681 in CYLD is essential for deubiquitinase activity. J Invest Dermatol. 2008;128:587–593. - PubMed
-
- Anderson DE, Howell JB. Epithelioma adenoides cysticum: Genetic update. Br J Dermatol. 1976;95:225–232. - PubMed
-
- Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, Dave S, Hurt EM, Tan B, Zhao H, Stephens O, Santra M, Williams DR, Dang L, Barlogie B, Shaughnessy JD, Jr, Kuehl WM, Staudt LM. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

