Evaluation of the transport, in vitro metabolism and pharmacokinetics of Salvinorin A, a potent hallucinogen
- PMID: 19462483
- PMCID: PMC2719774
- DOI: 10.1016/j.ejpb.2009.01.002
Evaluation of the transport, in vitro metabolism and pharmacokinetics of Salvinorin A, a potent hallucinogen
Abstract
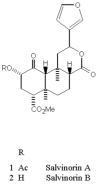
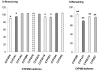
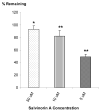
Salvinorin A is an unregulated potent hallucinogen isolated from the leaves of Salvia divinorum. It is the only known non-nitrogenous kappa-opioid selective agonist, and rivals synthetic lysergic acid diethylamide (LSD) in potency. The objective of this study was to characterize the in vitro transport, in vitro metabolism, and pharmacokinetic properties of Salvinorin A. The transport characteristics of Salvinorin A were assessed using MDCK-MDR1 cell monolayers. The P-glycoprotein (P-gp) affinity status was assessed by the P-gp ATPase assay. In vitro metabolism studies were performed with various specific human CYP450 isoforms and UGT2B7 to assess the metabolic characteristics of Salvinorin A. Cohorts (n = 3) of male Sprague Dawley rats were used to evaluate the pharmacokinetics and brain distribution of Salvinorin A (10 mg/kg, intraperitoneal (i.p.) over a 240-min period. A validated UV-HPLC and LC/MS/MS method was used to quantify the hallucinogen concentrations obtained from the in vitro and in vivo studies, respectively. Salvinorin A displayed a high secretory transport in the MDCK-MDR1 cells (4.07 +/- 1.34 x 10(-)5 cm/s). Salvinorin A also stimulated the P-gp ATPase activity in a concentration (5 and 10 microM)-dependent manner, suggesting that it may be a substrate of (P-gp). A significant decrease in Salvinorin A concentration ranging from 14.7 +/- 0.80% to 31.1 +/- 1.20% was observed after incubation with CYP2D6, CYP1A1, CYP2C18, and CYP2E1, respectively. A significant decrease was also observed after incubation with UGT2B7. These results suggest that Salvinorin A maybe a substrate of UGT2B7, CYP2D6, CYP1A1, CYP2E1, and CYP2C18. The in vivo pharmacokinetic study showed a relatively fast elimination with a half-life (t1/2) of 75 min and a clearance (Cl/F) of 26 L/h/kg. The distribution was extensive (Vd of 47.1 L/kg); however, the brain to plasma ratio was 0.050. Accordingly, the brain half-life was relatively short, 36 min. Salvinorin A is rapidly eliminated after i.p. dosing, in accordance with its fast onset and short duration of action. Further, it appears to be a substrate for various oxidative enzymes and multi-drug resistant protein, P-gp.
Figures

Similar articles
-
Pharmacokinetics of the potent hallucinogen, salvinorin A in primates parallels the rapid onset and short duration of effects in humans.Neuroimage. 2008 Jul 1;41(3):1044-50. doi: 10.1016/j.neuroimage.2008.03.003. Epub 2008 Mar 15. Neuroimage. 2008. PMID: 18434204 Free PMC article.
-
Behavioral effects and central nervous system levels of the broadly available κ-agonist hallucinogen salvinorin A are affected by P-glycoprotein modulation in vivo.J Pharmacol Exp Ther. 2012 Jun;341(3):802-8. doi: 10.1124/jpet.112.193227. Epub 2012 Mar 20. J Pharmacol Exp Ther. 2012. PMID: 22434677 Free PMC article.
-
Salvinorin B derivatives, EOM-Sal B and MOM-Sal B, produce stimulus generalization in male Sprague-Dawley rats trained to discriminate salvinorin A.Behav Pharmacol. 2011 Sep;22(5-6):450-7. doi: 10.1097/FBP.0b013e328349fc1b. Behav Pharmacol. 2011. PMID: 21814135
-
Salvinorin A: the "magic mint" hallucinogen finds a molecular target in the kappa opioid receptor.Trends Pharmacol Sci. 2003 Mar;24(3):107-9. doi: 10.1016/S0165-6147(03)00027-0. Trends Pharmacol Sci. 2003. PMID: 12628350 Review.
-
Neuropharmacology of the naturally occurring kappa-opioid hallucinogen salvinorin A.Pharmacol Rev. 2011 Jun;63(2):316-47. doi: 10.1124/pr.110.003244. Epub 2011 Mar 28. Pharmacol Rev. 2011. PMID: 21444610 Free PMC article. Review.
Cited by
-
Kappa Opioid Receptor Agonist and Brain Ischemia.Transl Perioper Pain Med. 2014;1(2):27-34. Transl Perioper Pain Med. 2014. PMID: 25574482 Free PMC article.
-
Emerging Psychotropic Drug for the Treatment of Trigeminal Pain: Salvinorin A.Pharmaceuticals (Basel). 2024 Nov 30;17(12):1619. doi: 10.3390/ph17121619. Pharmaceuticals (Basel). 2024. PMID: 39770461 Free PMC article. Review.
-
Salvinorin A and derivatives: protection from metabolism does not prolong short-term, whole-brain residence.Neuropharmacology. 2009 Sep;57(4):386-91. doi: 10.1016/j.neuropharm.2009.06.044. Epub 2009 Jul 8. Neuropharmacology. 2009. PMID: 19591852 Free PMC article.
-
Salvinorin A decreases mortality and improves neurological outcome in a neonatal mouse hypoxia model.Transl Perioper Pain Med. 2014;1(2):9-13. Transl Perioper Pain Med. 2014. PMID: 25568887 Free PMC article. No abstract available.
-
Immediate and Persistent Effects of Salvinorin A on the Kappa Opioid Receptor in Rodents, Monitored In Vivo with PET.Neuropsychopharmacology. 2015 Dec;40(13):2865-72. doi: 10.1038/npp.2015.159. Epub 2015 Jun 10. Neuropsychopharmacology. 2015. PMID: 26058662 Free PMC article.
References
-
- Giroud C, Felber F, Augsburger M, Horisberger B, Rivier L, Mangin P. Salvia divinorum: An hallucinogenic mint which might become a new recreational drug in Switzerland. Forensic Sci. Int. 2000;112:143–150. - PubMed
-
- Valdes LJ., 3rd Salvia divinorum and the unique diterpene hallucinogen, Salvinorin A (divinorin) J. Psychoactive Drugs. 1994;26:277–283. - PubMed
-
- Prisinzano TE. Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci. 2005;78:527–531. - PubMed
-
- Vortherms TA, Roth BL. Salvinorin A: From natural product to human theraputics. Molecular Interventions. 2006;6:257–265. - PubMed
-
- Grudmann O, Phipps SM, Zadezensky I, Butterweck V. Salvia divinorum and Salvinorin A: An update on pharmacology and analytical methodology. Planta Med. 2007;73:1039–1046. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

