The gastrointestinal tract and AIDS pathogenesis
- PMID: 19462506
- PMCID: PMC3755960
- DOI: 10.1053/j.gastro.2008.12.071
The gastrointestinal tract and AIDS pathogenesis
Abstract
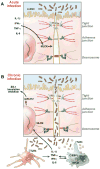
Gastrointestinal disease has been recognized as a major manifestation of human immunodeficiency virus infection since the earliest recognition of acquired immunodeficiency syndrome (AIDS). Originally, these disease manifestations were considered to be sequelae of the immune destruction that characterizes AIDS rather than being central to the pathogenesis of AIDS. Over time, it has become clear that the mucosal immune system in general and the intestinal immune system in particular are central to the pathogenesis of AIDS, with most of the critical events (eg, transmission, viral amplification, CD4+ T-cell destruction) occurring in the gastrointestinal tract. Compared with peripheral blood, these tissues are not easily accessible for analysis and have only begun to be examined in detail recently. In addition, although the resulting disease can progress over years, many critical events happen within the first few weeks of infection, when most patients are unaware that they are infected. Moreover, breakdown of the mucosal barrier and resulting microbial translocation are believed to be major drivers of AIDS progression. In this review, we focus on the interaction between primate lentiviruses and the gastrointestinal tract and discuss how this interaction promotes the pathogenesis of AIDS and drives immune dysfunction and progression to AIDS. This article draws extensively on work done in the nonhuman primate model of AIDS to fill gaps in our understanding of AIDS in humans.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures


References
-
- Alkhatib G, Combadiere C, Broder CC, et al. CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Moore JP, Kitchen SG, Pugach P, et al. The CCR5 and CXCR4 coreceptors—central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 2004;20:111–126. - PubMed
-
- Koff WC, Johnson PR, Watkins DI, et al. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006;7:19–23. - PubMed
-
- Picker LJ, Watkins DI. HIV pathogenesis: the first cut is the deepest. Nat Immunol. 2005;6:430–432. - PubMed
-
- Veazey RS, DeMaria M, Chalifoux LV, et al. The gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

