Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era
- PMID: 19462507
- PMCID: PMC4108289
- DOI: 10.1053/j.gastro.2009.01.072
Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era
Abstract
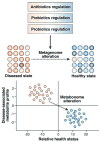
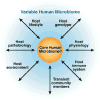
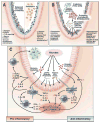
Studies of metagenomics and the human microbiome will tremendously expand our knowledge of the composition of microbial communities in the human body. As our understanding of microbial variation and corresponding genetic parameters is refined, this information can be applied to rational remodeling or "tailoring" of human-associated microbial communities and their associated functions. Physiologic features such as the development of innate and adaptive immunity, relative susceptibilities to infections, immune tolerance, bioavailability of nutrients, and intestinal barrier function may be modified by changing the composition and functions of the microbial communities. The specialty of gastroenterology will be affected profoundly by the ability to modify the gastrointestinal microbiota through the rational deployment of antibiotics, probiotics, and prebiotics. Antibiotics might be used to remove or suppress undesirable components of the human microbiome. Probiotics can introduce missing microbial components with known beneficial functions for the human host. Prebiotics can enhance the proliferation of beneficial microbes or probiotics, to maximize sustainable changes in the human microbiome. Combinations of these approaches might provide synergistic and effective therapies for specific disorders. The human microbiome could be manipulated by such "smart" strategies to prevent and treat acute gastroenteritis, antibiotic-associated diarrhea and colitis, inflammatory bowel disease, irritable bowel syndrome, necrotizing enterocolitis, and a variety of other disorders.
Conflict of interest statement
Conflicts of interest
The authors disclose the following: Dr. Versalovic has received unrestricted research support from Biogaia AB, Stockholm, Sweden, and has received honoraria from Group Danone, Paris, France.
Figures
Similar articles
-
Probiotics, prebiotics and the gastrointestinal tract in health and disease.Inflammopharmacology. 2014 Jun;22(3):135-54. doi: 10.1007/s10787-014-0201-4. Epub 2014 Mar 16. Inflammopharmacology. 2014. PMID: 24633989 Review.
-
Pre- and probiotic overview.Curr Opin Pharmacol. 2018 Dec;43:87-92. doi: 10.1016/j.coph.2018.08.010. Epub 2018 Sep 13. Curr Opin Pharmacol. 2018. PMID: 30219638 Review.
-
Potential role of the intestinal microbiota in programming health and disease.Nutr Rev. 2015 Aug;73 Suppl 1:32-40. doi: 10.1093/nutrit/nuv039. Nutr Rev. 2015. PMID: 26175488 Review.
-
The intestinal microbiome, probiotics and prebiotics in neurogastroenterology.Gut Microbes. 2013 Jan-Feb;4(1):17-27. doi: 10.4161/gmic.22973. Epub 2012 Nov 30. Gut Microbes. 2013. PMID: 23202796 Free PMC article. Review.
-
Antibiotics, probiotics and prebiotics in IBD.Nestle Nutr Inst Workshop Ser. 2014;79:83-100. doi: 10.1159/000360713. Epub 2014 Sep 5. Nestle Nutr Inst Workshop Ser. 2014. PMID: 25227297 Review.
Cited by
-
Intestinal microbiota, probiotics and human gastrointestinal cancers.J Gastrointest Cancer. 2013 Jun;44(2):121-31. doi: 10.1007/s12029-012-9459-1. J Gastrointest Cancer. 2013. Retraction in: J Gastrointest Cancer. 2013 Dec;44(4):491. doi: 10.1007/s12029-013-9542-2. PMID: 23180023 Retracted. Review.
-
Helicobacter pylori Related Diseases and Osteoporotic Fractures (Narrative Review).J Clin Med. 2020 Oct 12;9(10):3253. doi: 10.3390/jcm9103253. J Clin Med. 2020. PMID: 33053671 Free PMC article. Review.
-
Links between Nutrition, Infectious Diseases, and Microbiota: Emerging Technologies and Opportunities for Human-Focused Research.Nutrients. 2020 Jun 19;12(6):1827. doi: 10.3390/nu12061827. Nutrients. 2020. PMID: 32575399 Free PMC article. Review.
-
Antibiotics increase gut metabolism and antioxidant proteins and decrease acute phase response and necrotizing enterocolitis in preterm neonates.PLoS One. 2012;7(9):e44929. doi: 10.1371/journal.pone.0044929. Epub 2012 Sep 13. PLoS One. 2012. PMID: 23028687 Free PMC article.
-
Alterations in the Intestinal Morphology, Gut Microbiota, and Trace Mineral Status Following Intra-Amniotic Administration (Gallus gallus) of Teff (Eragrostis tef) Seed Extracts.Nutrients. 2020 Oct 2;12(10):3020. doi: 10.3390/nu12103020. Nutrients. 2020. PMID: 33023112 Free PMC article.
References
-
- Pennisi E. Metagenomics. Massive microbial sequence project proposed. Science. 2007;315:1781. - PubMed
-
- Backhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
-
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

