Measurement of monovalent and polyvalent carbohydrate-lectin binding by back-scattering interferometry
- PMID: 19462965
- PMCID: PMC2713007
- DOI: 10.1021/ac900569c
Measurement of monovalent and polyvalent carbohydrate-lectin binding by back-scattering interferometry
Abstract
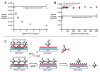
Carbohydrate-protein binding is important to many areas of biochemistry. Here, backscattering interferometry (BSI) has been shown to be a convenient and sensitive method for obtaining quantitative information about the strengths and selectivities of such interactions. The surfaces of glass microfluidic channels were covalently modified with extravidin, to which biotinylated lectins were subsequently attached by incubation and washing. The binding of unmodified carbohydrates to the resulting avidin-immobilized lectins was monitored by BSI. Dose-response curves that were generated within several minutes and were highly reproducible in multiple wash/measure cycles provided adsorption coefficients that showed mannose to bind to concanavalin A (conA) with 3.7 times greater affinity than glucose consistent with literature values. Galactose was observed to bind selectively and with similar affinity to the lectin BS-1. The avidities of polyvalent sugar-coated virus particles for immobilized conA were much higher than monovalent glycans, with increases of 60-200 fold per glycan when arrayed on the exterior surface of cowpea mosaic virus or bacteriophage Qbeta. Sugar-functionalized PAMAM dendrimers showed size-dependent adsorption, which was consistent with the expected density of lectins on the surface. The sensitivity of BSI matches or exceeds that of surface plasmon resonance and quartz crystal microbalance techniques, and is sensitive to the number of binding events, rather than changes in mass. The operational simplicity and generality of BSI, along with the near-native conditions under which the target binding proteins are immobilized, make BSI an attractive method for the quantitative characterization of the binding functions of lectins and other proteins.
Figures





References
-
- Lee YC, Lee RT. Accounts Chem Res. 1995;28:321–327.
-
- Lis H, Sharon N. Chem Rev. 1998;98:637–674. - PubMed
-
- Bertozzi CR, Kiessling LL. Science. 2001;291:2357–2364. - PubMed
-
- Collins BE, Paulson JC. Curr Opin Chem Biol. 2004;8:1–9. - PubMed
-
- Gabius HJ, Siebert HC, Andre S, Jimenez-Barbero J, Rudiger H. ChemBioChem. 2004;5:740–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

