Persistence of histone H2AX phosphorylation after meiotic chromosome synapsis and abnormal centromere cohesion in poly (ADP-ribose) polymerase (Parp-1) null oocytes
- PMID: 19463809
- PMCID: PMC2738933
- DOI: 10.1016/j.ydbio.2009.05.550
Persistence of histone H2AX phosphorylation after meiotic chromosome synapsis and abnormal centromere cohesion in poly (ADP-ribose) polymerase (Parp-1) null oocytes
Abstract
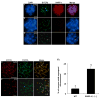
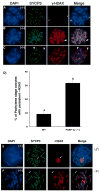
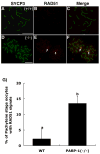
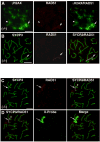
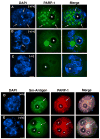
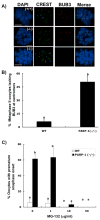
In spite of the impact of aneuploidy on human health little is known concerning the molecular mechanisms involved in the formation of structural or numerical chromosome abnormalities during meiosis. Here, we provide novel evidence indicating that lack of PARP-1 function during oogenesis predisposes the female gamete to genome instability. During prophase I of meiosis, a high proportion of Parp-1((-/-)) mouse oocytes exhibit a spectrum of meiotic defects including incomplete homologous chromosome synapsis or persistent histone H2AX phosphorylation in fully synapsed chromosomes at the late pachytene stage. Moreover, the X chromosome bivalent is also prone to exhibit persistent double strand DNA breaks (DSBs). In striking contrast, such defects were not detected in mutant pachytene spermatocytes. In fully-grown wild type oocytes at the germinal vesicle stage, PARP-1 protein associates with nuclear speckles and upon meiotic resumption, undergoes a striking re-localization towards spindle poles as well as pericentric heterochromatin domains at the metaphase II stage. Notably, a high proportion of in vivo matured Parp-1((-/-)) oocytes show lack of recruitment of the kinetochore-associated protein BUB3 to centromeric domains and fail to maintain metaphase II arrest. Defects in chromatin modifications in the form of persistent histone H2AX phosphorylation during prophase I of meiosis and deficient sister chromatid cohesion during metaphase II predispose mutant oocytes to premature anaphase II onset upon removal from the oviductal environment. Our results indicate that PARP-1 plays a critical role in the maintenance of chromosome stability at key stages of meiosis in the female germ line. Moreover, in the metaphase II stage oocyte PARP-1 is required for the regulation of centromere structure and function through a mechanism that involves the recruitment of BUB3 protein to centromeric domains.
Figures


Similar articles
-
Meiotic events at the centromeric heterochromatin: histone H3 phosphorylation, topoisomerase II alpha localization and chromosome condensation.Chromosoma. 1999 Dec;108(7):412-25. doi: 10.1007/s004120050393. Chromosoma. 1999. PMID: 10654080
-
Loss of maternal ATRX results in centromere instability and aneuploidy in the mammalian oocyte and pre-implantation embryo.PLoS Genet. 2010 Sep 23;6(9):e1001137. doi: 10.1371/journal.pgen.1001137. PLoS Genet. 2010. PMID: 20885787 Free PMC article.
-
Helicase LSH/Hells regulates kinetochore function, histone H3/Thr3 phosphorylation and centromere transcription during oocyte meiosis.Nat Commun. 2020 Sep 8;11(1):4486. doi: 10.1038/s41467-020-18009-3. Nat Commun. 2020. PMID: 32900989 Free PMC article.
-
Chromatin structure and ATRX function in mouse oocytes.Results Probl Cell Differ. 2012;55:45-68. doi: 10.1007/978-3-642-30406-4_3. Results Probl Cell Differ. 2012. PMID: 22918800 Review.
-
The molecular control of meiotic chromosomal behavior: events in early meiotic prophase in Drosophila oocytes.Annu Rev Physiol. 2012;74:425-51. doi: 10.1146/annurev-physiol-020911-153342. Annu Rev Physiol. 2012. PMID: 22335798 Review.
Cited by
-
Protective effects of a SIRT1 inhibitor on primordial follicle activation and growth induced by cyclophosphamide: insights from a bovine in vitro folliculogenesis system.J Assist Reprod Genet. 2022 Apr;39(4):933-943. doi: 10.1007/s10815-022-02437-9. Epub 2022 Mar 5. J Assist Reprod Genet. 2022. PMID: 35247119 Free PMC article.
-
Quantitative microscopy uncovers ploidy changes during mitosis in live Drosophila embryos and their effect on nuclear size.Biol Open. 2017 Mar 15;6(3):390-401. doi: 10.1242/bio.022079. Biol Open. 2017. PMID: 28108477 Free PMC article.
-
A Molecular Perspective and Role of NAD+ in Ovarian Aging.Int J Mol Sci. 2024 Apr 25;25(9):4680. doi: 10.3390/ijms25094680. Int J Mol Sci. 2024. PMID: 38731898 Free PMC article. Review.
-
Mitotic functions of poly(ADP-ribose) polymerases.Biochem Pharmacol. 2019 Sep;167:33-43. doi: 10.1016/j.bcp.2019.03.028. Epub 2019 Mar 22. Biochem Pharmacol. 2019. PMID: 30910692 Free PMC article. Review.
-
NAD+ depletion is central to placental dysfunction in an inflammatory subclass of preeclampsia.Life Sci Alliance. 2024 Oct 10;7(12):e202302505. doi: 10.26508/lsa.202302505. Print 2024 Dec. Life Sci Alliance. 2024. PMID: 39389781 Free PMC article.
References
-
- Ahel I, Ahel D, Matsusaka T, Clark AJ, Pines J, Boulton SJ, West SC. Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature. 2008;451:81–5. - PubMed
-
- Althaus FRHL, Kleczkowska HE, Malanga M, Naegeli H, Panzeter PL, Realini CA. Histone shuttling by poly ADP-ribosylation. Mol Cell Biochem. 1994;138:53–9. - PubMed
-
- Ame JC, Spelenhauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–93. - PubMed
-
- Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie DM, Monell C, Arnheim N, Bradley A, Ashley T, Liskay RM. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13:336–42. - PubMed
-
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

