Characterization of putative GRP- and NMB-receptor antagonist's interaction with human receptors
- PMID: 19463875
- PMCID: PMC2766550
- DOI: 10.1016/j.peptides.2009.05.007
Characterization of putative GRP- and NMB-receptor antagonist's interaction with human receptors
Abstract
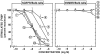
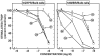
The mammalian bombesin (Bn) peptides neuromedin B (NMB) and gastrin-releasing peptide (GRP) actions are mediated by two receptors (NMB-receptor, GRP-receptor) which are widely distributed in the GI tract and CNS. From primarily animal studies NMB/GRP-receptor activation has physiological/pathophysiological effects in the CNS and GI tract including stimulating of growth of cancers and normal tissues. Whereas these Bn-receptors' effects have been extensively studied in nonhuman cells and animals, little is known of the physiological/pathological role(s) in humans, largely due to lack of potent antagonists. To address this issue we compared NMB/GRP-receptor affinity/potency of 10 chemical classes of putative antagonists (35 compounds) for human Bn-receptors by performing binding studies or assessing abilities to activate hGRP/hNMB-receptor [assessing phospholipase C activation] in four different cells containing native Bn-receptors or transfected receptors. From binding studies 23 were GRP-receptor-preferring, 4 were NMB-receptor, and 8 nonselective. For the hGRP-receptor-preferring analogues none showed hGRP-receptor agonist activity, but 13 were full or partial hNMB-receptor agonists at hNMB-receptors. For hNMB-receptor-preferring analogues none were agonists. Analogue #24 ([(3-Ph-Pr(6)), His(7), d-Ala(11), d-Pro(13), Psi(13-14), Phe(14)]Bn(6-14)NH2) and analogue #7 [d-Phe(6), Leu(13), Psi(CH(2)NH), Cpa(14)]Bn(6-14) were the most potent (0.2-1.4nM) and selective (>10,000-fold) for the hGRP-receptor with analogue #7.5 [d-Tpi(6), Leu(13), Psi(CH2NH), Leu(14)]Bn(6-14)[RC-3095] (0.2-1.4nM) slightly less selective. Analogue #34 (PD168368) had the highest affinity for hNMB-receptor (1.32-1.58nM) and the greatest selectivity (2298-6952-fold) for the hNMB-receptor. These results demonstrate numerous putative hGRP/hNMB-receptor antagonists identified in nonhuman cells and/or animals have agonist activity at the hNMB-receptor, limiting their potential usefulness. However, a number were identified which were potent/selective for human Bn-receptors and should be useful for investigating their roles in human physiological/pathophysiological conditions.
Figures
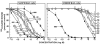

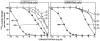
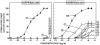
References
-
- Ashwood V, Brownhill V, Higginbottom M, Horwell DC, Hughes J, Lewthwaite RA, et al. PD 176252 - The first high affinity non-peptide gastrin-releasing peptide (BB2) receptor antagonist. Bioorg Med Chem. 1998;8:2589–94. - PubMed
-
- Benya RV, Fathi Z, Pradhan T, Battey JF, Kusui T, Jensen RT. Gastrin-releasing peptide receptor-induced internalization, down-regulation, desensitization and growth: Possible role of cAMP. Mol Pharmacol. 1994;46(2):235–45. - PubMed
-
- Benya RV, Kusui T, Pradhan TK, Battey JF, Jensen RT. Expression and characterization of cloned human bombesin receptors. Mol Pharmacol. 1995;47:10–20. - PubMed
-
- Benya RV, Wada E, Battey JF, Fathi Z, Wang LH, Mantey SA, et al. Neuromedin B receptors retain functional expression when transfected into BALB 3T3 fibroblasts: analysis of binding, kinetics, stoichiometry, modulation by guanine nucleotide-binding proteins, and signal transduction and comparison with natively expressed receptors. Mol Pharmacol. 1992;42(6):1058–68. - PubMed
-
- Corjay MH, Dobrzanski DJ, Way JM, Viallet J, Shapira H, Worland P, et al. Two distinct bombesin receptor subtypes are expressed and functional in human lung carcinoma cells. J Biol Chem. 1991;266:18771–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

