Lens aging: effects of crystallins
- PMID: 19463898
- PMCID: PMC2743770
- DOI: 10.1016/j.bbagen.2009.05.008
Lens aging: effects of crystallins
Abstract
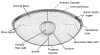
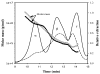
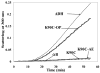
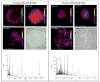
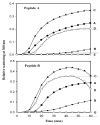
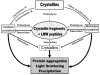
The primary function of the eye lens is to focus light on the retina. The major proteins in the lens--alpha, beta, and gamma-crystallins--are constantly subjected to age-related changes such as oxidation, deamidation, truncation, glycation, and methylation. Such age-related modifications are cumulative and affect crystallin structure and function. With time, the modified crystallins aggregate, causing the lens to increasingly scatter light on the retina instead of focusing light on it and causing the lens to lose its transparency gradually and become opaque. Age-related lens opacity, or cataract, is the major cause of blindness worldwide. We review deamidation, and glycation that occur in the lenses during aging keeping in mind the structural and functional changes that these modifications bring about in the proteins. In addition, we review proteolysis and discuss recent observations on how crystallin fragments generated in vivo, through their anti-chaperone activity may cause crystallin aggregation in aging lenses. We also review hyperbaric oxygen treatment induced guinea pig and 'humanized' ascorbate transporting mouse models as suitable options for studies on age-related changes in lens proteins.
Figures

References
-
- Dilley KJ, Pirie A. Changes to the proteins of the human lens nucleus in cataract. Exp Eye Res. 1974;19:59–72. - PubMed
-
- Spector A, Li S, Sigelman J. Age-dependent changes in the molecular size of human lens proteins and their relationship to light scatter. Invest Ophthalmol. 1974;13:795–798. - PubMed
-
- Bloemendal H. The lens proteins. In: Bloemendal H, editor. Molecular and Cellular Biology of the Eye Lens. New York: John Willey & Sons; 1981. pp. 1–49.
-
- Jaffe NS, Horwitz J. Lens and Cataract. In: Podos SM, Yanoff M, editors. Text Book of Ophthalmology. Vol. 3. Gower Med. Publishing; New York: 1991.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

