Fast inactivation of Shal (K(v)4) K+ channels is regulated by the novel interactor SKIP3 in Drosophila neurons
- PMID: 19463952
- PMCID: PMC2730949
- DOI: 10.1016/j.mcn.2009.05.003
Fast inactivation of Shal (K(v)4) K+ channels is regulated by the novel interactor SKIP3 in Drosophila neurons
Abstract
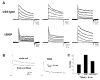
Shal K+ (K(v)4) channels across species carry the major A-type K+ current present in neurons. Shal currents are activated by small EPSPs and modulate post-synaptic potentials, backpropagation of action potentials, and induction of LTP. Fast inactivation of Shal channels regulates the impact of this post-synaptic modulation. Here, we introduce SKIP3, as the first protein interactor of Drosophila Shal K+ channels. The SKIP gene encodes three isoforms with multiple protein-protein interaction domains. SKIP3 is nervous system specific and co-localizes with Shal channels in neuronal cell bodies, and in puncta along processes. Using a genetic deficiency of SKIP, we show that the proportion of neurons displaying a very fast inactivation, consistent with Shal channels exclusively in a "fast" gating mode, is increased in the absence of SKIP3. As a scaffold-like protein, SKIP3 is likely to lead to the identification of a novel regulatory complex that modulates Shal channel inactivation.
Figures



Similar articles
-
Shal and shaker differential contribution to the K+ currents in the Drosophila mushroom body neurons.J Neurosci. 2005 Mar 2;25(9):2348-58. doi: 10.1523/JNEUROSCI.4384-04.2005. J Neurosci. 2005. PMID: 15745961 Free PMC article.
-
Shaker and Shal mediate transient calcium-independent potassium current in a Drosophila flight motoneuron.J Neurophysiol. 2009 Dec;102(6):3673-88. doi: 10.1152/jn.00693.2009. Epub 2009 Oct 14. J Neurophysiol. 2009. PMID: 19828724 Free PMC article.
-
Shal/K(v)4 channels are required for maintaining excitability during repetitive firing and normal locomotion in Drosophila.PLoS One. 2011 Jan 17;6(1):e16043. doi: 10.1371/journal.pone.0016043. PLoS One. 2011. PMID: 21264215 Free PMC article.
-
Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics.Nat Neurosci. 2010 Jan;13(1):53-9. doi: 10.1038/nn.2444. Epub 2009 Dec 6. Nat Neurosci. 2010. PMID: 19966842 Free PMC article.
-
Molecular physiology and modulation of somatodendritic A-type potassium channels.Mol Cell Neurosci. 2004 Dec;27(4):343-69. doi: 10.1016/j.mcn.2004.06.011. Mol Cell Neurosci. 2004. PMID: 15555915 Review.
Cited by
-
Putative excitatory and putative inhibitory inputs are localised in different dendritic domains in a Drosophila flight motoneuron.Eur J Neurosci. 2013 Mar;37(6):860-75. doi: 10.1111/ejn.12104. Epub 2012 Dec 27. Eur J Neurosci. 2013. PMID: 23279094 Free PMC article.
-
A screen for genes expressed in the olfactory organs of Drosophila melanogaster identifies genes involved in olfactory behaviour.PLoS One. 2012;7(4):e35641. doi: 10.1371/journal.pone.0035641. Epub 2012 Apr 18. PLoS One. 2012. PMID: 22530061 Free PMC article.
-
The mirtron miR-1010 functions in concert with its host gene SKIP to balance elevation of nAcRβ2.Sci Rep. 2020 Feb 3;10(1):1688. doi: 10.1038/s41598-020-58655-7. Sci Rep. 2020. PMID: 32015391 Free PMC article.
-
Age-related changes in Kv4/Shal and Kv1/Shaker expression in Drosophila and a role for reactive oxygen species.PLoS One. 2021 Dec 21;16(12):e0261087. doi: 10.1371/journal.pone.0261087. eCollection 2021. PLoS One. 2021. PMID: 34932577 Free PMC article.
-
Specialized eRpL22 paralogue-specific ribosomes regulate specific mRNA translation in spermatogenesis in Drosophila melanogaster.Mol Biol Cell. 2019 Aug 1;30(17):2240-2253. doi: 10.1091/mbc.E19-02-0086. Epub 2019 Jun 12. Mol Biol Cell. 2019. PMID: 31188709 Free PMC article.
References
-
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. - PubMed
-
- Brand A, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Brock MW, Lebaric ZN, Neumeister H, Detomaso A, Gilly WF. Temperature-dependent expression of a squid Kv1 channel in Sf9 cells and functional comparison with the native delayed rectifier. J Membr Biol. 2001;180:147–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

