NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation
- PMID: 19463978
- PMCID: PMC2732115
- DOI: 10.1016/j.bone.2009.05.009
NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation
Abstract
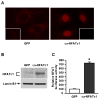
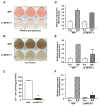
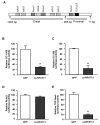
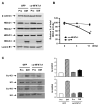
We previously reported that the in vivo and in vitro suppression of Nuclear Factor of Activated T Cells (NFAT) signaling increases osteoblast differentiation and bone formation. To investigate the mechanism by which NFATc1 regulates osteoblast differentiation, we established an osteoblast cell line that overexpresses a constitutively active NFATc1 (ca-NFATc1). The activation of NFATc1 significantly inhibits osteoblast differentiation and function, demonstrated by inhibition of alkaline phosphatase activity and mineralization as well as a decrease in gene expression of early and late markers of osteoblast differentiation such as osterix and osteocalcin, respectively. By focusing on the specific role of NFATc1 during late differentiation, we discovered that the inhibition of osteocalcin gene expression by NFATc1 was associated with a repression of the osteocalcin promoter activity, and a decrease in TCF/LEF transactivation. Also, overexpression of NFATc1 completely blocked the decrease in total histone deacetylase (HDAC) activity during osteoblast differentiation and prevented the hyperacetylation of histones H3 and H4. Mechanistically, we show by Chromatin Immunoprecipitation (ChIP) assay that the overexpression of NFATc1 sustains the binding of HDAC3 on the proximal region of the osteocalcin promoter, resulting in complete hypoacetylation of histones H3 and H4 when compared to GFP-expressing osteoblasts. In contrast, the inhibition of NFATc1 nuclear translocation either by cyclosporin or by using primary mouse osteoblasts with deleted calcineurin b1 prevents HDAC3 from associating with the proximal regulatory site of the osteocalcin promoter. These preliminary results suggest that NFATc1 acts as a transcriptional co-repressor of osteocalcin promoter, possibly in an HDAC-dependent manner.
Figures


Similar articles
-
Histone deacetylase 3 interacts with runx2 to repress the osteocalcin promoter and regulate osteoblast differentiation.J Biol Chem. 2004 Oct 1;279(40):41998-2007. doi: 10.1074/jbc.M403702200. Epub 2004 Aug 2. J Biol Chem. 2004. PMID: 15292260
-
Bone Morphogenetic Protein-2 (BMP-2) Activates NFATc1 Transcription Factor via an Autoregulatory Loop Involving Smad/Akt/Ca2+ Signaling.J Biol Chem. 2016 Jan 15;291(3):1148-61. doi: 10.1074/jbc.M115.668939. Epub 2015 Oct 15. J Biol Chem. 2016. PMID: 26472929 Free PMC article.
-
Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate.J Biol Chem. 2010 Aug 13;285(33):25251-8. doi: 10.1074/jbc.M110.110502. Epub 2010 Jun 16. J Biol Chem. 2010. PMID: 20554534 Free PMC article.
-
Osteocalcin gene promoter: unlocking the secrets for regulation of osteoblast growth and differentiation.J Cell Biochem Suppl. 1998;30-31:62-72. J Cell Biochem Suppl. 1998. PMID: 9893257 Review.
-
The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment.Crit Rev Oral Biol Med. 1999;10(1):40-57. doi: 10.1177/10454411990100010201. Crit Rev Oral Biol Med. 1999. PMID: 10759426 Review.
Cited by
-
PTH Signaling and Epigenetic Control of Bone Remodeling.Curr Mol Biol Rep. 2016 Mar;2(1):55-61. doi: 10.1007/s40610-016-0033-7. Epub 2016 Feb 3. Curr Mol Biol Rep. 2016. PMID: 27152252 Free PMC article.
-
Activation of Nfatc2 in osteoblasts causes osteopenia.J Cell Physiol. 2015 Jul;230(7):1689-95. doi: 10.1002/jcp.24928. J Cell Physiol. 2015. PMID: 25573264 Free PMC article.
-
Calcineurin activates interleukin-6 transcription in mouse skeletal muscle in vivo and in C2C12 myotubes in vitro.Am J Physiol Regul Integr Comp Physiol. 2010 Jan;298(1):R198-210. doi: 10.1152/ajpregu.00325.2009. Epub 2009 Nov 11. Am J Physiol Regul Integr Comp Physiol. 2010. PMID: 19907005 Free PMC article.
-
HDAC3 selectively represses CREB3-mediated transcription and migration of metastatic breast cancer cells.Cell Mol Life Sci. 2010 Oct;67(20):3499-510. doi: 10.1007/s00018-010-0388-5. Epub 2010 May 15. Cell Mol Life Sci. 2010. PMID: 20473547 Free PMC article.
-
Deletion of Orai1 leads to bone loss aggravated with aging and impairs function of osteoblast lineage cells.Bone Rep. 2018 Apr 5;8:147-155. doi: 10.1016/j.bonr.2018.03.007. eCollection 2018 Jun. Bone Rep. 2018. PMID: 29955633 Free PMC article.
References
-
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–32. - PubMed
-
- Yeo H, Beck LH, Thompson SR, Farach-Carson MC, McDonald JM, Clemens TL, Zayzafoon M. Conditional disruption of calcineurin b1 in osteoblasts increases bone formation and reduces bone resorption. J Biol Chem. 2007;282:35318–27. - PubMed
-
- Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res. 1992;7:683–92. - PubMed
-
- Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

