Structure-activity studies on the lycorine pharmacophore: A potent inducer of apoptosis in human leukemia cells
- PMID: 19464034
- PMCID: PMC7111684
- DOI: 10.1016/j.phytochem.2009.04.012
Structure-activity studies on the lycorine pharmacophore: A potent inducer of apoptosis in human leukemia cells
Abstract
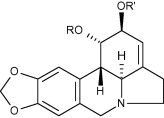
The direct chemoselective differential functionalization of the ring-C hydroxyl groups present in the Amaryllidaceae alkaloid lycorine is described allowing for selective manipulation of the 1,2-hydroxyl groups. A mini-library comprised of synthetic and natural lycorane alkaloids was prepared and their apoptosis-inducing activity investigated in human leukemia (Jurkat) cells. Further insights into the nature of this interesting apoptosis-inducing pharmacophore are described, including the requirement of both free hydroxyl groups in ring-C.
Figures

Similar articles
-
Cytotoxic lycorine alkaloids of the plant family Amaryllidaceae.Bioorg Chem. 2025 Aug;163:108619. doi: 10.1016/j.bioorg.2025.108619. Epub 2025 May 23. Bioorg Chem. 2025. PMID: 40516169 Review.
-
Structure activity studies on the crinane alkaloid apoptosis-inducing pharmacophore.Nat Prod Commun. 2009 Apr;4(4):483-8. Nat Prod Commun. 2009. PMID: 19475990
-
In search of a cytostatic agent derived from the alkaloid lycorine: synthesis and growth inhibitory properties of lycorine derivatives.Bioorg Med Chem. 2011 Dec 1;19(23):7252-61. doi: 10.1016/j.bmc.2011.09.051. Epub 2011 Oct 2. Bioorg Med Chem. 2011. PMID: 22019045 Free PMC article.
-
Lycorine and its derivatives for anticancer drug design.Mini Rev Med Chem. 2010 Jan;10(1):41-50. doi: 10.2174/138955710791112604. Mini Rev Med Chem. 2010. PMID: 20105122 Review.
-
Amaryllidaceae alkaloids belonging to different structural subgroups display activity against apoptosis-resistant cancer cells.J Nat Prod. 2010 Jul 23;73(7):1223-7. doi: 10.1021/np9008255. J Nat Prod. 2010. PMID: 20550100
Cited by
-
Design of semisynthetic derivatives of the Amaryllidaceae alkaloid ambelline and exploration of their in vitro cytotoxic activities.Saudi Pharm J. 2023 Aug;31(8):101684. doi: 10.1016/j.jsps.2023.06.017. Epub 2023 Jun 24. Saudi Pharm J. 2023. PMID: 37457365 Free PMC article.
-
Novel lycorine derivatives as anticancer agents: synthesis and in vitro biological evaluation.Molecules. 2014 Feb 21;19(2):2469-80. doi: 10.3390/molecules19022469. Molecules. 2014. PMID: 24566315 Free PMC article.
-
Uncover the anticancer potential of lycorine.Chin Med. 2024 Sep 8;19(1):121. doi: 10.1186/s13020-024-00989-9. Chin Med. 2024. PMID: 39245716 Free PMC article. Review.
-
Multiple biological functions and pharmacological effects of lycorine.Sci China Chem. 2013;56(10):1382-1391. doi: 10.1007/s11426-013-4967-9. Epub 2013 Aug 24. Sci China Chem. 2013. PMID: 32215001 Free PMC article. Review.
-
Lycorine: A prospective natural lead for anticancer drug discovery.Biomed Pharmacother. 2018 Nov;107:615-624. doi: 10.1016/j.biopha.2018.07.147. Epub 2018 Aug 14. Biomed Pharmacother. 2018. PMID: 30114645 Free PMC article. Review.
References
-
- Arrigoni O., Arrigoni Liso R., Calabrese G. Lycorine as an inhibitor of ascorbic acid biosynthesis. Nature. 1975;256:513–514.
-
- Campbell W.E., Nair J.J., Gammon D.W., Bastida J., Codina C., Viladomat F., Smith P.J., Albrecht C.F. Cyotoxic and antimalarial alkaloids from Brunsvigia littoralis. Planta Medica. 1998;64:91–93. - PubMed
-
- Citoglu G., Tanker M., Gamusel B. Antiinflammatory effects of lycorine and haemanthidine. Phytotherapy Research. 1998;12(3):205–206.
-
- De Leo P., Dalessandro G., De Santis A., Arrigoni O. Inhibitory effect of lycorine on cell division and cell elongation. Plant and Cell Physiology. 1973;14(3):481–486.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

