The extracellular domain of Smoothened regulates ciliary localization and is required for high-level Hh signaling
- PMID: 19464178
- PMCID: PMC2892286
- DOI: 10.1016/j.cub.2009.04.053
The extracellular domain of Smoothened regulates ciliary localization and is required for high-level Hh signaling
Abstract
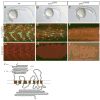
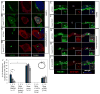
Members of the Hedgehog (Hh) family of secreted proteins function as morphogens to pattern developing tissues and control cell proliferation. The seven-transmembrane domain (7TM) protein Smoothened (Smo) is essential for the activation of all levels of Hh signaling. However, the mechanisms by which Smo differentially activates low- or high-level Hh signaling are not known. Here we show that a newly identified mutation in the extracellular domain (ECD) of zebrafish Smo attenuates Smo signaling. The Smo agonist purmorphamine induces the stabilization, ciliary translocation, and high-level signaling of wild-type Smo. In contrast, purmorphamine induces the stabilization but not the ciliary translocation or high-level signaling of the Smo ECD mutant protein. Surprisingly, a truncated form of Smo that lacks the cysteine-rich domain of the ECD localizes to the cilium but is unable to activate high-level Hh signaling. We also present evidence that cilia may be required for Hh signaling in early zebrafish embryos. These data indicate that the ECD, previously thought to be dispensable for vertebrate Smo function, both regulates Smo ciliary localization and is essential for high-level Hh signaling.
Figures


References
-
- Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. - PubMed
-
- Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2:29–30. - PubMed
-
- Ingham PW, Nystedt S, Nakano Y, Brown W, Stark D, van den Heuvel M, Taylor AM. Patched represses the hedgehog signalling pathway by promoting modification of the Smoothened protein. Curr Biol. 2000;10:1315–1318. - PubMed
-
- Zhao Y, Tong C, Jiang J. Hedgehog regulates smoothened activity by inducing a conformational change. Nature. 2007;450:252–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

