A DNA vaccine targeting the receptor-binding domain of Clostridium difficile toxin A
- PMID: 19464540
- PMCID: PMC2709243
- DOI: 10.1016/j.vaccine.2009.03.058
A DNA vaccine targeting the receptor-binding domain of Clostridium difficile toxin A
Abstract
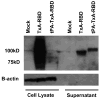
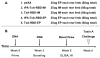
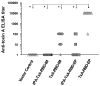
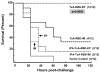
Clostridium difficile is a pathogen with increasing severity for which host antibody responses provide protection from disease. DNA vaccination has several advantages compared to traditional vaccine methods, however no study has examined this platform against C. difficile toxins. A synthetic gene was created encoding the receptor-binding domain (RBD) of C. difficile toxin A, optimized for expression in human cells. Gene expression was examined in vitro. Mice were inoculated and then challenged with parenteral toxin A. Vaccination provided high titer antibodies and protected mice from death. This represents the first report of DNA vaccine inducing neutralizing antibodies to C. difficile toxin A.
Figures



Similar articles
-
An optimized, synthetic DNA vaccine encoding the toxin A and toxin B receptor binding domains of Clostridium difficile induces protective antibody responses in vivo.Infect Immun. 2014 Oct;82(10):4080-91. doi: 10.1128/IAI.01950-14. Epub 2014 Jul 14. Infect Immun. 2014. PMID: 25024365 Free PMC article.
-
A chimeric toxin vaccine protects against primary and recurrent Clostridium difficile infection.Infect Immun. 2012 Aug;80(8):2678-88. doi: 10.1128/IAI.00215-12. Epub 2012 May 21. Infect Immun. 2012. PMID: 22615245 Free PMC article.
-
Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A.Infect Immun. 2003 Mar;71(3):1608-10. doi: 10.1128/IAI.71.3.1608-1610.2003. Infect Immun. 2003. PMID: 12595488 Free PMC article.
-
Against Clostridioides difficile Infection: An Update on Vaccine Development.Toxins (Basel). 2025 May 1;17(5):222. doi: 10.3390/toxins17050222. Toxins (Basel). 2025. PMID: 40423305 Free PMC article. Review.
-
Toxin-specific antibodies for the treatment of Clostridium difficile: current status and future perspectives.Toxins (Basel). 2010 May;2(5):998-1018. doi: 10.3390/toxins2050998. Epub 2010 May 7. Toxins (Basel). 2010. PMID: 22069622 Free PMC article. Review.
Cited by
-
A Review of Experimental and Off-Label Therapies for Clostridium difficile Infection.Infect Dis Ther. 2017 Mar;6(1):1-35. doi: 10.1007/s40121-016-0140-z. Epub 2016 Dec 1. Infect Dis Ther. 2017. PMID: 27910000 Free PMC article. Review.
-
Intravenous adenovirus expressing a multi-specific, single-domain antibody neutralizing TcdA and TcdB protects mice from Clostridium difficile infection.Pathog Dis. 2016 Oct;74(7):ftw078. doi: 10.1093/femspd/ftw078. Epub 2016 Aug 7. Pathog Dis. 2016. PMID: 27502696 Free PMC article.
-
Recombinant antigens based on toxins A and B of Clostridium difficile that evoke a potent toxin-neutralising immune response.Vaccine. 2014 Feb 3;32(6):700-5. doi: 10.1016/j.vaccine.2013.11.099. Epub 2013 Dec 14. Vaccine. 2014. PMID: 24342251 Free PMC article.
-
An optimized, synthetic DNA vaccine encoding the toxin A and toxin B receptor binding domains of Clostridium difficile induces protective antibody responses in vivo.Infect Immun. 2014 Oct;82(10):4080-91. doi: 10.1128/IAI.01950-14. Epub 2014 Jul 14. Infect Immun. 2014. PMID: 25024365 Free PMC article.
-
Toll-like receptor 5-dependent immunogenicity and protective efficacy of a recombinant fusion protein vaccine containing the nontoxic domains of Clostridium difficile toxins A and B and Salmonella enterica serovar typhimurium flagellin in a mouse model of Clostridium difficile disease.Infect Immun. 2013 Jun;81(6):2190-6. doi: 10.1128/IAI.01074-12. Epub 2013 Apr 1. Infect Immun. 2013. PMID: 23545305 Free PMC article.
References
-
- Yannelli B, Gurevich I, Schoch PE, Cunha BA. Yield of stool cultures, ova and parasite tests, and Clostridium difficile determinations in nosocomial diarrheas. Am J Infect Control. 1988;16(6):246–9. - PubMed
-
- Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. Jama. 1990;263(7):979–82. - PubMed
-
- Giannasca PJ, Warny M. Active and passive immunization against Clostridium difficile diarrhea and colitis. Vaccine. 2004;22(7):848–56. - PubMed
-
- McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

