Vaccination with vif-deleted feline immunodeficiency virus provirus, GM-CSF, and TNF-alpha plasmids preserves global CD4 T lymphocyte function after challenge with FIV
- PMID: 19464559
- PMCID: PMC2802579
- DOI: 10.1016/j.vaccine.2009.03.081
Vaccination with vif-deleted feline immunodeficiency virus provirus, GM-CSF, and TNF-alpha plasmids preserves global CD4 T lymphocyte function after challenge with FIV
Abstract
Feline immunodeficiency virus (FIV) DNA vaccine approaches that included a vif-deleted FIV provirus (FIV-pPPRDeltavif) and feline cytokine expression plasmids were tested for immunogenicity and efficacy by immunization of specific pathogen free cats. Vaccine protocols included FIV-pPPRDeltavif plasmid alone; a combination of FIV-pPPRDeltavif DNA and feline granulocyte macrophage-colony stimulating factor (GM-CSF) and tumor necrosis factor (TNF)-alpha expression plasmids; or a combination of FIV-pPPRDeltavif and feline interleukin (IL)-15 plasmids. Cats immunized with FIV-pPPRDeltavif, GM-CSF and TNF-alpha plasmids demonstrated an increased frequency of FIV-specific T cell proliferation responses compared to other vaccine groups. Immunization with FIV-pPPRDeltavif and IL-15 plasmids was distinguished from other vaccine protocols by the induction of antiviral antibodies. Suppression of virus loads was not observed for any of the FIV-pPPRDeltavif DNA vaccine protocols after challenge with the FIV-PPR isolate. However, prior immunization with FIV-pPPRDeltavif, GM-CSF, and TNF-alpha plasmids resulted in preservation of CD4 T cell functions, including mitogen-induced cytokine expression and antigen-specific proliferation upon infection with FIV. These findings justify further examination of cytokine combinations as adjuvants for lentiviral DNA vaccines.
Figures
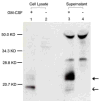



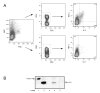
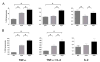
References
-
- Dunham SP. Lessons from cat: development of vaccines against lentiviruses. Vet Immunol Immunopathol. 2006;112(1–2):67–77. - PubMed
-
- Lecollinet S, Richardson J. Vaccination against the feline immunodeficiency virus: The road not taken. Comp Immunol Microbiol Infect Dis. 2008;31(2–3):167–90. - PubMed
-
- Boretti FS, Leutenegger CM, Mislin CN, Hofmann-Lehmann R, Koenig S, Schroff M, et al. Protection against FIV challenge infection by genetic vaccination using minimalistic DNA constructs for FIV env gene and feline IL-12 expression. AIDS. 2000;14(12):1749–57. - PubMed
-
- Dunham SP, Flynn JN, Rigby MA, Macdonald J, Bruce J, Cannon C, et al. Protection against feline immunodeficiency virus using replication defective proviral DNA vaccines with feline interleukin-12 and -18. Vaccine. 2002;20(11–12):1483–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

