Age-appropriate use of human papillomavirus vaccines in the U.S
- PMID: 19464729
- PMCID: PMC2729751
- DOI: 10.1016/j.ygyno.2009.04.035
Age-appropriate use of human papillomavirus vaccines in the U.S
Abstract
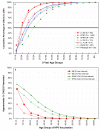
Cervical infections by approximately 15 cancer-associated (carcinogenic) human papillomavirus (HPV) genotypes cause virtually all cervical cancer and its immediate precursor lesions worldwide. Prophylactic vaccines against human papillomavirus (HPV) types HPV16 and HP18, which cause 70% of cervical cancer worldwide, hold great promise for reducing the burden of cervical cancer worldwide. However, current HPV vaccines prevent future infections and related cervical abnormalities and do not treat pre-existing HPV infections. In the U.S., HPV vaccine introduction should be considered in the context of a very successful cervical cancer screening program that has reduced the rates of cervical cancer by 75% or more. Thus, HPV vaccines will only prevent an incremental number of additional cervical cancers in the U.S. The introduction of HPV vaccines can also prevent other HPV-related sequelae, most importantly cervical intraepithelial neoplasia grade 2 or 3 (CIN2/3), which precede the development of cervical cancer and require clinical follow-up and treatment. Examining data from 7 clinical centers in the U.S., the median age of CIN2/3 is typically between 25 and 30 years of age in 2007; if screen-detected CIN2/3 develops on average 5-10 years after the causal infection is acquired, HPV vaccination will only prevent a significant proportion of CIN2/3 if it is given to women before the age of 26 and more so if given to women 18 and younger. It is increasingly evident that prophylactic HPV vaccines will provide the greatest public health or population benefit only when delivered to adolescent, mostly HPV-naive women.
Figures
References
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999 Sep;189(1):12–9. - PubMed
-
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003 Feb 6;348(6):518–27. - PubMed
-
- Schiffman MH, Bauer HM, Hoover RN, Glass AG, Cadell DM, Rush BB, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993 Jun 16;85(12):958–64. - PubMed
-
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007 Sep 8;370(9590):890–907. - PubMed
-
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007 Aug 1;121(3):621–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

