Control of regulatory T cell lineage commitment and maintenance
- PMID: 19464984
- PMCID: PMC4410181
- DOI: 10.1016/j.immuni.2009.04.009
Control of regulatory T cell lineage commitment and maintenance
Abstract
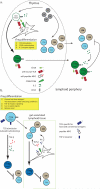
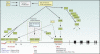
Foxp3-expressing regulatory T (Treg) cells suppress pathology mediated by immune responses against self and foreign antigens and commensal microorganisms. Sustained expression of the transcription factor Foxp3, a key distinguishing feature of Treg cells, is required for their differentiation and suppressor function. In addition, Foxp3 expression prevents deviation of Treg cells into effector T cell lineages and confers dependence of Treg cell survival and expansion on growth factors, foremost interleukin-2, provided by activated effector T cells. In this review we discuss Treg cell differentiation and maintenance with a particular emphasis on molecular regulation of Foxp3 expression, arguably a key to mechanistic understanding of biology of regulatory T cells.
Figures
References
-
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. - PubMed
-
- Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–5472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

