A complex of three related membrane proteins is conserved on malarial merozoites
- PMID: 19465059
- PMCID: PMC2724972
- DOI: 10.1016/j.molbiopara.2009.05.006
A complex of three related membrane proteins is conserved on malarial merozoites
Abstract
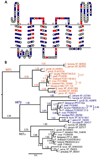
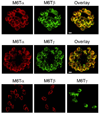
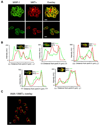
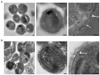
Invasion of human red blood cells by the malaria parasite Plasmodium falciparum is a coordinated, multi-step process. Here, we describe three novel integral membrane proteins that colocalize on the inner membrane complex immediately beneath the merozoite plasma membrane. Each has six predicted transmembrane domains and is conserved in diverse apicomplexan parasites. Immunoprecipitation studies using specific antibodies reveal that these proteins assemble into a heteromeric complex. Each protein was also expressed on insect cells using the baculovirus vector system with a truncated SUMO tag that facilitates maximal expression and protein purification while permitting cleavage with SUMO protease to release unmodified parasite protein. The expressed proteins were successfully reconstituted into artificial liposomes, but were not recognized by human immune sera. Because all three genes are highly conserved in apicomplexan parasites, the complex formed by their encoded proteins likely serves an essential role for invasive merozoites.
Figures


Similar articles
-
Plasmodium falciparum MSP3 Exists in a Complex on the Merozoite Surface and Generates Antibody Response during Natural Infection.Infect Immun. 2018 Jul 23;86(8):e00067-18. doi: 10.1128/IAI.00067-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29760216 Free PMC article.
-
A novel Pfs38 protein complex on the surface of Plasmodium falciparum blood-stage merozoites.Malar J. 2017 Feb 16;16(1):79. doi: 10.1186/s12936-017-1716-0. Malar J. 2017. PMID: 28202027 Free PMC article.
-
Characterization of Pv92, a Novel Merozoite Surface Protein of Plasmodium vivax.Korean J Parasitol. 2016 Aug;54(4):385-91. doi: 10.3347/kjp.2016.54.4.385. Epub 2016 Aug 31. Korean J Parasitol. 2016. PMID: 27658588 Free PMC article.
-
Apical membrane antigen 1 as an anti-malarial drug target.Curr Top Med Chem. 2011;11(16):2039-47. doi: 10.2174/156802611796575885. Curr Top Med Chem. 2011. PMID: 21619512 Review.
-
Antibodies and Plasmodium falciparum merozoites.Trends Parasitol. 2001 Apr;17(4):194-7. doi: 10.1016/s1471-4922(00)01946-2. Trends Parasitol. 2001. PMID: 11282510 Review.
Cited by
-
The alveolin IMC1h is required for normal ookinete and sporozoite motility behaviour and host colonisation in Plasmodium berghei.PLoS One. 2012;7(7):e41409. doi: 10.1371/journal.pone.0041409. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844474 Free PMC article.
-
The inner membrane complex sub-compartment proteins critical for replication of the apicomplexan parasite Toxoplasma gondii adopt a pleckstrin homology fold.J Biol Chem. 2014 May 16;289(20):13962-73. doi: 10.1074/jbc.M114.548891. Epub 2014 Mar 27. J Biol Chem. 2014. PMID: 24675080 Free PMC article.
-
An essential contractile ring protein controls cell division in Plasmodium falciparum.Nat Commun. 2019 May 16;10(1):2181. doi: 10.1038/s41467-019-10214-z. Nat Commun. 2019. PMID: 31097714 Free PMC article.
-
Detecting mutations in PfCRT and PfMDR1 genes among Plasmodium falciparum isolates from Saudi Arabia by pyrosequencing.Parasitol Res. 2011 Aug;109(2):291-6. doi: 10.1007/s00436-011-2251-5. Epub 2011 Feb 25. Parasitol Res. 2011. PMID: 21350795
-
Apicomplexan lineage-specific polytopic membrane proteins in Cryptosporidium parvum.J Parasit Dis. 2020 Jun;44(2):467-471. doi: 10.1007/s12639-020-01209-5. Epub 2020 Mar 13. J Parasit Dis. 2020. PMID: 32508425 Free PMC article.
References
-
- Gaur D, Mayer DC, Miller LH. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol. 2004;34:1413–1429. - PubMed
-
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. - PubMed
-
- Kappe SH, Buscaglia CA, Bergman LW, et al. Apicomplexan gliding motility and host cell invasion: overhauling the motor model. Trends Parasitol. 2004;20:13–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

