The formation and biological significance of N7-guanine adducts
- PMID: 19465146
- PMCID: PMC2739241
- DOI: 10.1016/j.mrgentox.2009.05.006
The formation and biological significance of N7-guanine adducts
Abstract
DNA alkylation or adduct formation occurs at nucleophilic sites in DNA, mainly the N7-position of guanine. Ever since identification of the first N7-guanine adduct, several hundred studies on DNA adducts have been reported. Major issues addressed include the relationships between N7-guanine adducts and exposure, mutagenesis, and other biological endpoints. It became quickly apparent that N7-guanine adducts are frequently formed, but may have minimal biological relevance, since they are chemically unstable and do not participate in Watson Crick base pairing. However, N7-guanine adducts have been shown to be excellent biomarkers for internal exposure to direct acting and metabolically activated carcinogens. Questions arise, however, regarding the biological significance of N7-guanine adducts that are readily formed, do not persist, and are not likely to be mutagenic. Thus, we set out to review the current literature to evaluate their formation and the mechanistic evidence for the involvement of N7-guanine adducts in mutagenesis or other biological processes. It was concluded that there is insufficient evidence that N7-guanine adducts can be used beyond confirmation of exposure to the target tissue and demonstration of the molecular dose. There is little to no evidence that N7-guanine adducts or their depurination product, apurinic sites, are the cause of mutations in cells and tissues, since increases in AP sites have not been shown unless toxicity is extant. However, more research is needed to define the extent of chemical depurination versus removal by DNA repair proteins. Interestingly, N7-guanine adducts are clearly present as endogenous background adducts and the endogenous background amounts appear to increase with age. Furthermore, the N7-guanine adducts have been shown to convert to ring opened lesions (FAPy), which are much more persistent and have higher mutagenic potency. Studies in humans are limited in sample size and differences between controls and study groups are small. Future investigations should involve human studies with larger numbers of individuals and analysis should include the corresponding ring opened FAPy derivatives.
Figures
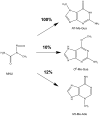



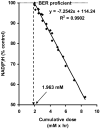

References
-
- Reiner B, Zamenhof S. Studies on the chemically reactive groups of deoxyribonucleic acids. J Biol Chem. 1957;228:475–486. - PubMed
-
- Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953;171:737–738. - PubMed
-
- Boysen G, Hecht SS. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutat Res. 2003;543:17–30. - PubMed
-
- van Zeeland AA. Molecular dosimetry of chemical mutagens. Relationship between DNA adduct formation and genetic changes analyzed at the molecular level. Mutat Res. 1996;353:123–150. - PubMed
-
- De Bont R, Van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

