TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity
- PMID: 19465477
- PMCID: PMC2740458
- DOI: 10.1074/jbc.M109.010264
TDP-43 is intrinsically aggregation-prone, and amyotrophic lateral sclerosis-linked mutations accelerate aggregation and increase toxicity
Erratum in
- J Biol Chem. 2009 Sep 11;284(37):25459
Abstract
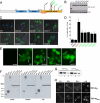
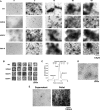
Non-amyloid, ubiquitinated cytoplasmic inclusions containing TDP-43 and its C-terminal fragments are pathological hallmarks of amyotrophic lateral sclerosis (ALS), a fatal motor neuron disorder, and frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U). Importantly, TDP-43 mutations are linked to sporadic and non-SOD1 familial ALS. However, TDP-43 is not the only protein in disease-associated inclusions, and whether TDP-43 misfolds or is merely sequestered by other aggregated components is unclear. Here, we report that, in the absence of other components, TDP-43 spontaneously forms aggregates bearing remarkable ultrastructural similarities to TDP-43 deposits in degenerating neurons of ALS and FTLD-U patients [corrected] . The C-terminal domain of TDP-43 is critical for spontaneous aggregation. Several ALS-linked TDP-43 mutations within this domain (Q331K, M337V, Q343R, N345K, R361S, and N390D) increase the number of TDP-43 aggregates and promote toxicity in vivo. Importantly, mutations that promote toxicity in vivo accelerate aggregation of pure TDP-43 in vitro. Thus, TDP-43 is intrinsically aggregation-prone, and its propensity for toxic misfolding trajectories is accentuated by specific ALS-linked mutations.
Figures



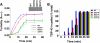

References
-
- Ayala Y. M., Pantano S., D'Ambrogio A., Buratti E., Brindisi A., Marchetti C., Romano M., Baralle F. E. (2005) J. Mol. Biol. 348, 575–588 - PubMed
-
- Buratti E., Baralle F. E. (2001) J. Biol. Chem. 276, 36337–36343 - PubMed
-
- Neumann M., Sampathu D. M., Kwong L. K., Truax A. C., Micsenyi M. C., Chou T. T., Bruce J., Schuck T., Grossman M., Clark C. M., McCluskey L. F., Miller B. L., Masliah E., Mackenzie I. R., Feldman H., Feiden W., Kretzschmar H. A., Trojanowski J. Q., Lee V. M. (2006) Science 314, 130–133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

