Binding modes of aromatic ligands to mammalian heme peroxidases with associated functional implications: crystal structures of lactoperoxidase complexes with acetylsalicylic acid, salicylhydroxamic acid, and benzylhydroxamic acid
- PMID: 19465478
- PMCID: PMC2740456
- DOI: 10.1074/jbc.M109.010280
Binding modes of aromatic ligands to mammalian heme peroxidases with associated functional implications: crystal structures of lactoperoxidase complexes with acetylsalicylic acid, salicylhydroxamic acid, and benzylhydroxamic acid
Abstract
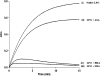
The binding and structural studies of bovine lactoperoxidase with three aromatic ligands, acetylsalicylic acid (ASA), salicylhydoxamic acid (SHA), and benzylhydroxamic acid (BHA) show that all the three compounds bind to lactoperoxidase at the substrate binding site on the distal heme side. The binding of ASA occurs without perturbing the position of conserved heme water molecule W-1, whereas both SHA and BHA displace it by the hydroxyl group of their hydroxamic acid moieties. The acetyl group carbonyl oxygen atom of ASA forms a hydrogen bond with W-1, which in turn makes three other hydrogen-bonds, one each with heme iron, His-109 N(epsilon2), and Gln-105 N(epsilon2). In contrast, in the complexes of SHA and BHA, the OH group of hydroxamic acid moiety in both complexes interacts with heme iron directly with Fe-OH distances of 3.0 and 3.2A respectively. The OH is also hydrogen bonded to His-109 N(epsilon2) and Gln-105N(epsilon2). The plane of benzene ring of ASA is inclined at 70.7 degrees from the plane of heme moiety, whereas the aromatic planes of SHA and BHA are nearly parallel to the heme plane with inclinations of 15.7 and 6.2 degrees , respectively. The mode of ASA binding provides the information about the mechanism of action of aromatic substrates, whereas the binding characteristics of SHA and BHA indicate the mode of inhibitor binding.
Figures




Similar articles
-
Mode of binding of the tuberculosis prodrug isoniazid to heme peroxidases: binding studies and crystal structure of bovine lactoperoxidase with isoniazid at 2.7 A resolution.J Biol Chem. 2010 Jan 8;285(2):1569-76. doi: 10.1074/jbc.M109.060327. Epub 2009 Nov 11. J Biol Chem. 2010. PMID: 19907057 Free PMC article.
-
Binding of salicylhydroxamic acid and several aromatic donor molecules to Arthromyces ramosus peroxidase, investigated by X-ray crystallography, optical difference spectroscopy, NMR relaxation, molecular dynamics, and kinetics.Biochemistry. 1999 Sep 28;38(39):12558-68. doi: 10.1021/bi982925l. Biochemistry. 1999. PMID: 10504224
-
Inhibition of lactoperoxidase by its own catalytic product: crystal structure of the hypothiocyanate-inhibited bovine lactoperoxidase at 2.3-A resolution.Biophys J. 2009 Jan;96(2):646-54. doi: 10.1016/j.bpj.2008.09.019. Biophys J. 2009. PMID: 19167310 Free PMC article.
-
Structural basis of activation of mammalian heme peroxidases.Prog Biophys Mol Biol. 2018 Mar;133:49-55. doi: 10.1016/j.pbiomolbio.2017.11.003. Epub 2017 Nov 22. Prog Biophys Mol Biol. 2018. PMID: 29174286 Review.
-
Structural studies on bovine heart cytochrome c oxidase.Biochim Biophys Acta. 2012 Apr;1817(4):579-89. doi: 10.1016/j.bbabio.2011.12.012. Epub 2012 Jan 4. Biochim Biophys Acta. 2012. PMID: 22236806 Review.
Cited by
-
First structural evidence for the mode of diffusion of aromatic ligands and ligand-induced closure of the hydrophobic channel in heme peroxidases.J Biol Inorg Chem. 2010 Sep;15(7):1099-107. doi: 10.1007/s00775-010-0669-3. Epub 2010 May 12. J Biol Inorg Chem. 2010. PMID: 20461536
-
Mode of binding of the tuberculosis prodrug isoniazid to heme peroxidases: binding studies and crystal structure of bovine lactoperoxidase with isoniazid at 2.7 A resolution.J Biol Chem. 2010 Jan 8;285(2):1569-76. doi: 10.1074/jbc.M109.060327. Epub 2009 Nov 11. J Biol Chem. 2010. PMID: 19907057 Free PMC article.
-
Structure of Yak Lactoperoxidase at 1.55 Å Resolution.Protein J. 2021 Feb;40(1):8-18. doi: 10.1007/s10930-020-09957-2. Epub 2021 Jan 3. Protein J. 2021. PMID: 33389415
-
Dual binding mode of antithyroid drug methimazole to mammalian heme peroxidases - structural determination of the lactoperoxidase-methimazole complex at 1.97 Å resolution.FEBS Open Bio. 2016 Jun 14;6(7):640-50. doi: 10.1002/2211-5463.12051. eCollection 2016 Jul. FEBS Open Bio. 2016. PMID: 27398304 Free PMC article.
-
Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Thiouracil Derivatives as Potential Antithyroid Agents.Molecules. 2018 Nov 8;23(11):2913. doi: 10.3390/molecules23112913. Molecules. 2018. PMID: 30413058 Free PMC article.
References
-
- Furtmüller P. G., Zederbauer M., Jantschko W., Helm J., Bogner M., Jakopitsch C., Obinger C. (2006) Arch. Biochem. Biophys. 445, 199–213 - PubMed
-
- Poulos T. L., Edwards S. L., Wariishi H., Gold M. H. (1993) J. Biol. Chem. 268, 4429–4440 - PubMed
-
- Gajhede M. (2001) Biochem. Soc. Trans. 29, 91–98 - PubMed
-
- Sievers G. (1981) FEBS Lett. 127, 253–256 - PubMed
-
- Cals M. M., Mailliart P., Brignon G., Anglade P., Dumas B. R. (1991) Eur. J. Biochem. 198, 733–739 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

