Interleukin-33 is biologically active independently of caspase-1 cleavage
- PMID: 19465481
- PMCID: PMC2740567
- DOI: 10.1074/jbc.M901744200
Interleukin-33 is biologically active independently of caspase-1 cleavage
Abstract
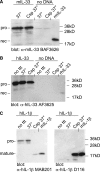
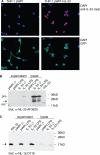
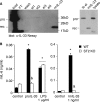
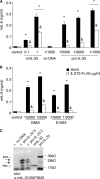
The new interleukin (IL)-1 family cytokine IL-33 is synthesized as a 30-kDa precursor. Like pro-IL-1beta, human pro-IL-33 was reported to be cleaved by caspase-1 to generate an 18-kDa fragment, which is sufficient to activate signaling by the IL-33 receptor T1/ST2. However, the proposed caspase-1 cleavage site is poorly conserved between species. In addition, it is not clear whether caspase-1 cleavage of pro-IL-33 occurs in vivo and whether, as for IL-1beta, this cleavage is a prerequisite for IL-33 secretion and bioactivity. In this study, we further investigated caspase-1 cleavage of mouse and human pro-IL-33 and assessed the potential bioactivity of the IL-33 precursor. We observed the generation of a 20-kDa IL-33 fragment in cell lysates, which was enhanced by incubation with caspase-1. However, in vitro assays of mouse and human pro-IL-33 indicated that IL-33 is not a direct substrate for this enzyme. Consistently, caspase-1 activation in THP-1 cells induced cleavage of pro-IL-1beta but not of pro-IL-33, and activated THP-1 cells released full-length pro-IL-33 into culture supernatants. Finally, addition of full-length pro-IL-33 induced T1/ST2-dependent IL-6 secretion in mast cells. However, we observed in situ processing of pro-IL-33 in mast cell cultures, and it remains to be determined whether full-length pro-IL-33 itself indeed represents the bioactive species. In conclusion, our data indicate that pro-IL-33 is not a direct substrate for caspase-1. In addition, our results clearly show that caspase-1 cleavage is not required for pro-IL-33 secretion and bioactivity, highlighting major differences between IL-1beta and IL-33.
Figures

References
-
- Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T. K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D. M., Bazan J. F., Kastelein R. A. (2005) Immunity 23, 479–490 - PubMed
-
- Howard A. D., Kostura M. J., Thornberry N., Ding G. J., Limjuco G., Weidner J., Salley J. P., Hogquist K. A., Chaplin D. D., Mumford R. A., Schmidt J. A., Tocci M. J. (1991) J. Immunol. 147, 2964–2969 - PubMed
-
- Lüschen S., Ussat S., Krönke M., Adam-Klages S. (1998) Biochem. Biophys. Res. Commun. 253, 92–98 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

