The contribution of inositol 1,4,5-trisphosphate and ryanodine receptors to agonist-induced Ca(2+) signaling of airway smooth muscle cells
- PMID: 19465516
- PMCID: PMC2742787
- DOI: 10.1152/ajplung.90559.2008
The contribution of inositol 1,4,5-trisphosphate and ryanodine receptors to agonist-induced Ca(2+) signaling of airway smooth muscle cells
Abstract
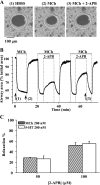
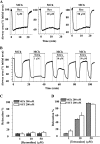
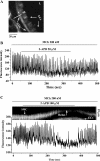
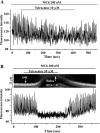
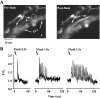
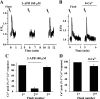
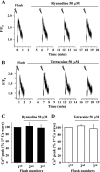
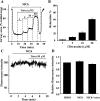
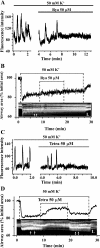
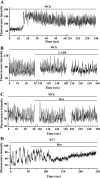
The relative contribution of inositol 1,4,5-trisphosphate (IP(3)) receptors (IP(3)Rs) and ryanodine receptors (RyRs) to agonist-induced Ca(2+) signaling in mouse airway smooth muscle cells (SMCs) was investigated in lung slices with phase-contrast or laser scanning microscopy. At room temperature (RT), methacholine (MCh) or 5-hydroxytryptamine (5-HT) induced Ca(2+) oscillations and an associated contraction in small airway SMCs. The subsequent exposure to an IP(3)R antagonist, 2-aminoethoxydiphenyl borate (2-APB), inhibited the Ca(2+) oscillations and induced airway relaxation in a concentration-dependent manner. 2-APB also inhibited Ca(2+) waves generated by the photolytic release of IP(3). However, the RyR antagonist ryanodine had no significant effect, at any concentration, on airway contraction or agonist- or IP(3)-induced Ca(2+) oscillations or Ca(2+) wave propagation. By contrast, a second RyR antagonist, tetracaine, relaxed agonist-contracted airways and inhibited agonist-induced Ca(2+) oscillations in a concentration-dependent manner. However, tetracaine did not affect IP(3)-induced Ca(2+) release or wave propagation nor the Ca(2+) content of SMC Ca(2+) stores as evaluated by Ca(2+)-release induced by caffeine. Conversely, both ryanodine and tetracaine completely blocked agonist-independent slow Ca(2+) oscillations induced by KCl. The inhibitory effects of 2-APB and absence of an effect of ryanodine on MCh-induced airway contraction or Ca(2+) oscillations of SMCs were also observed at 37 degrees C. In Ca(2+)-permeable SMCs, tetracaine inhibited agonist-induced contraction without affecting intracellular Ca(2+) levels indicating that relaxation also resulted from a reduction in Ca(2+) sensitivity. These results indicate that agonist-induced Ca(2+) oscillations in mouse small airway SMCs are primary mediated via IP(3)Rs and that tetracaine induces relaxation by both decreasing Ca(2+) sensitivity and inhibiting agonist-induced Ca(2+) oscillations via an IP(3)-dependent mechanism.
Figures




References
-
- Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L909–L917, 2004. - PubMed
-
- Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol 291: L208–L221, 2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

