Apoptosis predominates in nonmyocytes in heart failure
- PMID: 19465551
- PMCID: PMC2724204
- DOI: 10.1152/ajpheart.00310.2009
Apoptosis predominates in nonmyocytes in heart failure
Abstract
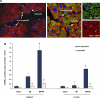
The goal of this investigation was to determine the distribution of myocardial apoptosis in myocytes and nonmyocytes in primates and patients with heart failure (HF). Almost all clinical cardiologists and cardiovascular investigators believe that myocyte apoptosis is considered to be a cardinal sign of HF and a major factor in its pathogenesis. However, with the knowledge that 75% of the number of cells in the heart are nonmyocytes, it is important to determine whether the apoptosis in HF is occurring in myocytes or in nonmyocytes. We studied both a nonhuman primate model of chronic HF, induced by rapid pacing 2-6 mo after myocardial infarction (MI), and biopsies from patients with ischemic cardiomyopathy. Dual labeling with a cardiac muscle marker was used to discriminate apoptosis in myocytes versus nonmyocytes. Left ventricular ejection fraction decreased following MI (from 78% to 60%) and further with HF (35%, P < 0.05). As expected, total apoptosis was increased in the myocardium following recovery from MI (0.62 cells/mm(2)) and increased further with the development of HF (1.91 cells/mm(2)). Surprisingly, the majority of apoptotic cells in MI and MI + HF, and in both the adjacent and remote areas, were nonmyocytes. This was also observed in myocardial biopsies from patients with ischemic cardiomyopathy. We found that macrophages contributed the largest fraction of apoptotic nonmyocytes (41% vs. 18% neutrophils, 16% fibroblast, and 25% endothelial and other cells). Although HF in the failing human and monkey heart is characterized by significant apoptosis, in contrast to current concepts, the apoptosis in nonmyocytes was eight- to ninefold greater than in myocytes.
Figures



Similar articles
-
Cardiac macrophages and apoptosis after myocardial infarction: effects of central MR blockade.Am J Physiol Regul Integr Comp Physiol. 2014 Oct 1;307(7):R879-87. doi: 10.1152/ajpregu.00075.2014. Epub 2014 Aug 6. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 25100076
-
Apoptosis in severe, compensated pressure overload predominates in nonmyocytes and is related to the hypertrophy but not function.Am J Physiol Heart Circ Physiol. 2011 Mar;300(3):H1062-8. doi: 10.1152/ajpheart.00998.2010. Epub 2010 Dec 10. Am J Physiol Heart Circ Physiol. 2011. PMID: 21148760 Free PMC article.
-
Renal denervation improves cardiac function by attenuating myocardiocyte apoptosis in dogs after myocardial infarction.BMC Cardiovasc Disord. 2018 May 8;18(1):86. doi: 10.1186/s12872-018-0828-y. BMC Cardiovasc Disord. 2018. PMID: 29739333 Free PMC article.
-
[Cell death in inflammatory heart muscle diseases--apoptosis or necrosis?].Herz. 1999 May;24(3):211-8. doi: 10.1007/BF03044963. Herz. 1999. PMID: 10412644 Review. German.
-
Anti-apoptosis in nonmyocytes and pro-autophagy in cardiomyocytes: two strategies against postinfarction heart failure through regulation of cell death/degeneration.Heart Fail Rev. 2018 Sep;23(5):759-772. doi: 10.1007/s10741-018-9708-x. Heart Fail Rev. 2018. PMID: 29737434 Review.
Cited by
-
Programmed Cell Death of Endothelial Cells in Myocardial Infarction and Its Potential Therapeutic Strategy.Cardiol Res Pract. 2022 May 11;2022:6558060. doi: 10.1155/2022/6558060. eCollection 2022. Cardiol Res Pract. 2022. PMID: 35600331 Free PMC article. Review.
-
Cardiac telocytes inhibit cardiac microvascular endothelial cell apoptosis through exosomal miRNA-21-5p-targeted cdip1 silencing to improve angiogenesis following myocardial infarction.Theranostics. 2021 Jan 1;11(1):268-291. doi: 10.7150/thno.47021. eCollection 2021. Theranostics. 2021. PMID: 33391474 Free PMC article.
-
The Role of microRNAs in Heart Failure: A Systematic Review.Front Cardiovasc Med. 2020 Oct 15;7:161. doi: 10.3389/fcvm.2020.00161. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 33195446 Free PMC article.
-
Truncations of the titin Z-disc predispose to a heart failure with preserved ejection phenotype in the context of pressure overload.PLoS One. 2018 Jul 31;13(7):e0201498. doi: 10.1371/journal.pone.0201498. eCollection 2018. PLoS One. 2018. PMID: 30063764 Free PMC article.
-
Interplay of Oxidative Stress and Necrosis-like Cell Death in Cardiac Ischemia/Reperfusion Injury: A Focus on Necroptosis.Biomedicines. 2022 Jan 7;10(1):127. doi: 10.3390/biomedicines10010127. Biomedicines. 2022. PMID: 35052807 Free PMC article. Review.
References
-
- Abbate A, Biondi-Zoccai GG, Baldi A. Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol 193: 145–153, 2002. - PubMed
-
- Akasaka Y, Morimoto N, Ishikawa Y, Fujita K, Ito K, Kimura-Matsumoto M, Ishiguro S, Morita H, Kobayashi Y, Ishii T. Myocardial apoptosis associated with the expression of proinflammatory cytokines during the course of myocardial infarction. Mod Pathol 19: 588–598, 2006. - PubMed
-
- Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, Natividad F, Bishop SP, Vatner SF. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol 20: 1493–1499, 2000. - PubMed
-
- Asai K, Kudej RK, Takagi G, Kudej AB, Natividad F, Shen YT, Vatner DE, Vatner SF. Paradoxically enhanced endothelin-B receptor-mediated vasoconstriction in conscious old monkeys. Circulation 103: 2382–2386, 2001. - PubMed
-
- Baldi A, Abbate A, Bussani R, Patti G, Melfi R, Angelini A, Dobrina A, Rossiello R, Silvestri F, Baldi F, Di Sciascio G. Apoptosis and post-infarction left ventricular remodeling. J Mol Cell Cardiol 34: 165–174, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

