Regulation of gap junctional charge selectivity in cells coexpressing connexin 40 and connexin 43
- PMID: 19465552
- PMCID: PMC2711728
- DOI: 10.1152/ajpheart.00287.2009
Regulation of gap junctional charge selectivity in cells coexpressing connexin 40 and connexin 43
Abstract
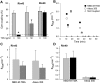
Expression of connexin 40 (Cx40) and Cx43 in cardiovascular tissues varies as a function of age, injury, and development with unknown consequences on the selectivity of junctional communication and its acute regulation. We investigated the PKC-dependent regulation of charge selectivity in junctions composed of Cx43, Cx40, or both by simultaneous assessment of junctional permeance rate constants (B(dye)) for dyes of similar size but opposite charge, N,N,N-trimethyl-2-[methyl-(7-nitro-2,1,3-benzoxadiol-4-yl)amino]ethanaminium (NBD-M-TMA; +1) and Alexa 350 (-1). The ratio of dye rate constants (B(NBD-M-TMA)/B(Alexa 350)) indicated that Cx40 junctions are cation selective (10.7 +/- 0.5), whereas Cx43 junction are nonselective (1.22 +/- 0.14). In coexpressing cells, a broad range of junctional selectivities was observed with mean cation selectivity increasing as the Cx40 to Cx43 expression ratio increased. PKC activation reduced or eliminated dye permeability of Cx43 junctions without altering their charge selectivity, had no effect on either permeability or charge selectivity of Cx40 junctions, and significantly increased the cation selectivity of junctions formed by coexpressing cells (approaching charge selectivity of Cx40 junctions). Junctions composed of Cx43 truncated at residue 257 (Cx43tr) were also not charge selective, but when Cx43tr was coexpressed with Cx40, a broad range of junctional selectivities that was unaffected by PKC activation was observed. Thus, whereas the charge selectivities of homomeric/homotypic Cx43 and Cx40 junctions appear invariant, the selectivities of junctions formed by cells coexpressing Cx40 and Cx43 vary considerably, reflecting both their relative expression levels and phosphorylation-dependent regulation. Such regulation could represent a mechanism by which coexpressing cells such as vascular endothelium and atrial cells regulate acutely the selective intercellular communication mediated by their gap junctions.
Figures
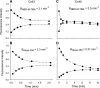
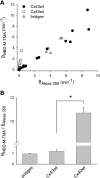

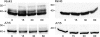
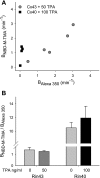
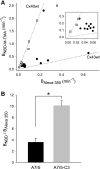
 ) and A7r5 (•) cell junctions (dotted lines are the linear regression fits for Cx40 and Cx43 junctions as shown in Fig. 2A). Inset shows data near graph origin at expanded scaling of both axes. B: the average charge selectivities (BNBD-M-TMA/BAlexa 350) of A7r5 and A7r5-C3 junctions are significantly different, P < <0.001. *Selectivity of A7r5-C3 junctions significantly greater than A7r5 junctions (Student's t-test).
) and A7r5 (•) cell junctions (dotted lines are the linear regression fits for Cx40 and Cx43 junctions as shown in Fig. 2A). Inset shows data near graph origin at expanded scaling of both axes. B: the average charge selectivities (BNBD-M-TMA/BAlexa 350) of A7r5 and A7r5-C3 junctions are significantly different, P < <0.001. *Selectivity of A7r5-C3 junctions significantly greater than A7r5 junctions (Student's t-test).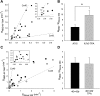

References
-
- Aavula BR, Ahad Ali M, Mash EA, Bednarczyk D, Wright SH. Synthesis and fluorescence of N,N,N-trimethyl-2-[methyl(7-nitrobenzo[c][l,2,5]oxadiazol-4-yl) amino]ethanaminium iodide, a pH-insensitive reporter of organic cation transport. Synth Commun 36: 701–705, 2006.
-
- Bolon ML, Kidder GM, Simon AM, Tyml K. Lipopolysaccharide reduces electrical coupling in microvascular endothelial cells by targeting connexin40 in a tyrosine-, ERK1/2-, PKA-, and PKC-dependent manner. J Cell Physiol 211: 159–166, 2007. - PubMed
-
- Bolon ML, Peng T, Kidder GM, Tyml K. Lipopolysaccharide plus hypoxia and reoxygenation synergistically reduce electrical coupling between microvascular endothelial cells by dephosphorylating connexin40. J Cell Physiol 217: 350–359, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

