The Patched dependence receptor triggers apoptosis through a DRAL-caspase-9 complex
- PMID: 19465923
- PMCID: PMC2844407
- DOI: 10.1038/ncb1880
The Patched dependence receptor triggers apoptosis through a DRAL-caspase-9 complex
Abstract
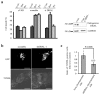
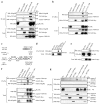
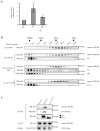
Sonic hedgehog (Shh) and its main receptor, Patched (Ptc), are implicated in both neural development and tumorigenesis. Besides its classic morphogenic activity, Shh is also a survival factor. Along this line, Ptc has been shown to function as a dependence receptor; it induces apoptosis in the absence of Shh, whereas its pro-apoptotic activity is blocked in the presence of Shh. Here we show that, in the absence of its ligand, Ptc interacts with the adaptor protein DRAL (downregulated in rhabdomyosarcoma LIM-domain protein; also known as FHL2). DRAL is required for the pro-apoptotic activity of Ptc both in immortalized cells and during neural tube development in chick embryos. We demonstrate that, in the absence of Shh, Ptc recruits a protein complex that includes DRAL, one of the caspase recruitment (CARD)-domain containing proteins TUCAN (family member, 8) or NALP1 (NLR family, pyrin domain containing 1) and apical caspase-9. Ptc triggers caspase-9 activation and enhances cell death through a caspase-9-dependent mechanism. Thus, we propose that in the absence of its ligand Shh the dependence receptor Ptc serves as the anchor for a caspase-activating complex that includes DRAL, and caspase-9.
Figures


References
-
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. - PubMed
-
- Murone M, Rosenthal A, de Sauvage FJ. Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr Biol. 1999;9:76–84. - PubMed
-
- Charrier JB, Lapointe F, Le Douarin NM, Teillet MA. Anti-apoptotic role of Sonic hedgehog protein at the early stages of nervous system organogenesis. Development. 2001;128:4011–4020. - PubMed
-
- Litingtung Y, Chiang C. Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat Neurosci. 2000;3:979–985. - PubMed
-
- Thibert C, et al. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

