Protective mechanisms of activated protein C in severe inflammatory disorders
- PMID: 19466992
- PMCID: PMC2785525
- DOI: 10.1111/j.1476-5381.2009.00251.x
Protective mechanisms of activated protein C in severe inflammatory disorders
Abstract
The protein C system is an important natural anticoagulant mechanism mediated by activated protein C (APC) that regulates the activity of factors VIIIa and Va. Besides well-defined anticoagulant properties, APC also demonstrates anti-inflammatory, anti-apoptotic and endothelial barrier-stabilizing effects that are collectively referred to as the cytoprotective effects of APC. Many of these beneficial effects are mediated through its co-receptor endothelial protein C receptor, and the protease-activated receptor 1, although exact mechanisms remain unclear and are likely pleiotropic in nature. Increased insight into the structure-function relationships of APC facilitated design of APC variants that conserve cytoprotective effects and reduce anticoagulant features, thereby attenuating the risk of severe bleeding with APC therapy. Impairment of the protein C system plays an important role in acute lung injury/acute respiratory distress syndrome and severe sepsis. The pathophysiology of both diseases states involves uncontrolled inflammation, enhanced coagulation and compromised fibrinolysis. This leads to microvascular thrombosis and organ injury. Administration of recombinant human APC to correct the dysregulated protein C system reduced mortality in severe sepsis patients (PROWESS trial), which stimulated further research into its mechanisms of action. Several other clinical trials evaluating recombinant human APC have been completed, including studies in children and less severely ill adults with sepsis as well as a study in acute lung injury. On the whole, these studies have not supported the use of APC in these populations and challenge the field of APC research to search for additional answers.
Figures
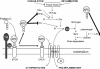
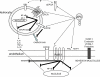
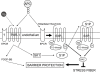
References
-
- Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290:238–247. - PubMed
-
- Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353:1332–1341. - PubMed
-
- Aoki Y, Ota M, Katsuura Y, Komoriya K, Nakagaki T. Effect of activated human protein C on disseminated intravascular coagulation induced by lipopolysaccharide in rats. Arzneimittelforschung. 2000;50:809–815. - PubMed
-
- Bae JS, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007a;110:3909–3916. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

