Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications
- PMID: 19467172
- PMCID: PMC2878476
- DOI: 10.1017/S1462399409001082
Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications
Abstract
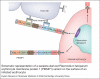
Severe malaria has a high mortality rate (15-20%) despite treatment with effective antimalarial drugs. Adjunctive therapies for severe malaria that target the underlying disease process are therefore urgently required. Adhesion of erythrocytes infected with Plasmodium falciparum to human cells has a key role in the pathogenesis of life-threatening malaria and could be targeted with antiadhesion therapy. Parasite adhesion interactions include binding to endothelial cells (cytoadherence), rosetting with uninfected erythrocytes and platelet-mediated clumping of infected erythrocytes. Recent research has started to define the molecular mechanisms of parasite adhesion, and antiadhesion therapies are being explored. However, many fundamental questions regarding the role of parasite adhesion in severe malaria remain unanswered. There is strong evidence that rosetting contributes to severe malaria in sub-Saharan Africa; however, the identity of other parasite adhesion phenotypes that are implicated in disease pathogenesis remains unclear. In addition, the possibility of geographic variation in adhesion phenotypes causing severe malaria, linked to differences in malaria transmission levels and host immunity, has been neglected. Further research is needed to realise the untapped potential of antiadhesion adjunctive therapies, which could revolutionize the treatment of severe malaria and reduce the high mortality rate of the disease.
Figures


Comment in
-
Red blood cell complement receptor one level varies with Knops blood group, α(+)thalassaemia and age among Kenyan children.Genes Immun. 2016 Apr;17(3):171-8. doi: 10.1038/gene.2016.2. Epub 2016 Feb 4. Genes Immun. 2016. PMID: 26844958 Free PMC article.
References
-
- Marsh K.. et al. Indicators of life-threatening malaria in African children. New England Journal of Medicine. 1995;332:1399–1404. - PubMed
-
- Dondorp A.M.. et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clinical Infectious Diseases. 2008;47:151–157. - PubMed
-
- Miller L.H.. et al. The pathogenic basis of malaria. Nature. 2002;415:673–679. - PubMed
-
- Dondorp A.M.. et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. Journal of Infectious Diseases. 2008;197:79–84. - PubMed
Further reading, resources and contacts
Books
-
- D.A. Warrell, H.M. Gilles. 4th Edition. Oxford University Press; 2002. eds ( ) Essential Malariology,
-
This book provides a broad background to malariology.
-
- I.W. Sherman. ASM Press; 2005. ed. ( ) Molecular Approaches to Malaria,
-
A good overview of current molecular approaches to malaria research.
Websites
-
- http://www.cdc.gov/Malaria/ http://www.cdc.gov/Malaria/
-
For general information on malaria:
-
- http://www.who.int/topics/malaria/en/ http://www.who.int/topics/malaria/en/
-
For information on malaria parasite biology, biochemistry and physiology see:
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

