The effect of endocrine responsiveness on high-risk breast cancer treated with dose-intensive chemotherapy: results of International Breast Cancer Study Group Trial 15-95 after prolonged follow-up
- PMID: 19468030
- PMCID: PMC2720817
- DOI: 10.1093/annonc/mdp024
The effect of endocrine responsiveness on high-risk breast cancer treated with dose-intensive chemotherapy: results of International Breast Cancer Study Group Trial 15-95 after prolonged follow-up
Abstract
Background: The role of adjuvant dose-intensive chemotherapy and its efficacy according to baseline features has not yet been established.
Patients and methods: Three hundred and forty-four patients were randomized to receive seven courses of standard-dose chemotherapy (SD-CT) or three cycles of dose-intensive epirubicin and cyclophosphamide (epirubicin 200 mg/m(2) plus cyclophosphamide 4 mg/m(2) with filgrastim and progenitor cell support). All patients were assigned tamoxifen at the completion of chemotherapy. The primary end point was disease-free survival (DFS). This paper updates the results and explores patterns of recurrence according to predicting baseline features.
Results: At 8.3-years median follow-up, patients assigned DI-EC had a significantly better DFS compared with those assigned SD-CT [8-year DFS percent 47% and 37%, respectively, hazard ratio (HR) 0.76; 95% confidence interval 0.58-1.00; P = 0.05]. Only patients with estrogen receptor (ER)-positive disease benefited from the DI-EC (HR 0.61; 95% confidence interval 0.39, 0.95; P = 0.03).
Conclusions: After prolonged follow-up, DI-EC significantly improved DFS, but the effect was observed only in patients with ER-positive disease, leading to the hypothesis that efficacy of DI-EC may relate to its endocrine effects. Further studies designed to confirm the importance of endocrine responsiveness in patients treated with dose-intensive chemotherapy are encouraged.
Figures
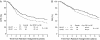

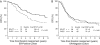

Similar articles
-
Multicycle dose-intensive chemotherapy for women with high-risk primary breast cancer: results of International Breast Cancer Study Group Trial 15-95.J Clin Oncol. 2006 Jan 20;24(3):370-8. doi: 10.1200/JCO.2005.03.5196. J Clin Oncol. 2006. PMID: 16421418 Clinical Trial.
-
Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study.J Clin Oncol. 2003 Mar 1;21(5):843-50. doi: 10.1200/JCO.2003.05.135. J Clin Oncol. 2003. PMID: 12610183 Review.
-
Multicycle high-dose chemotherapy and filgrastim-mobilized peripheral-blood progenitor cells in women with high-risk stage II or III breast cancer: five-year follow-up.J Clin Oncol. 1999 Jan;17(1):82-92. doi: 10.1200/JCO.1999.17.1.82. J Clin Oncol. 1999. PMID: 10458221 Clinical Trial.
-
Randomised trial: survival benefit and safety of adjuvant dose-dense chemotherapy for node-positive breast cancer.Br J Cancer. 2006 May 8;94(9):1237-44. doi: 10.1038/sj.bjc.6603085. Br J Cancer. 2006. PMID: 16622463 Free PMC article. Clinical Trial.
-
Adjuvant chemotherapy in early breast cancer.Dan Med J. 2016 May;63(5):B5222. Dan Med J. 2016. PMID: 27127018 Review.
Cited by
-
Changes in ovarian function in premenopausal women with breast cancer undergoing adjuvant TC (docetaxel and cyclophosphamide) chemotherapy during a brief period of amenorrhea around the last chemotherapy cycle.Springerplus. 2014 Jul 10;3:352. doi: 10.1186/2193-1801-3-352. eCollection 2014. Springerplus. 2014. PMID: 25077063 Free PMC article.
-
Aurora-A mitotic kinase induces endocrine resistance through down-regulation of ERα expression in initially ERα+ breast cancer cells.PLoS One. 2014 May 9;9(5):e96995. doi: 10.1371/journal.pone.0096995. eCollection 2014. PLoS One. 2014. PMID: 24816249 Free PMC article.
-
High-Dose Chemotherapy With Hematopoietic Stem Cell Transplant in Patients With High-Risk Breast Cancer and 4 or More Involved Axillary Lymph Nodes: 20-Year Follow-up of a Phase 3 Randomized Clinical Trial.JAMA Oncol. 2020 Apr 1;6(4):528-534. doi: 10.1001/jamaoncol.2019.6276. JAMA Oncol. 2020. PMID: 31999296 Free PMC article. Clinical Trial.
-
Raf-1 oncogenic signaling is linked to activation of mesenchymal to epithelial transition pathway in metastatic breast cancer cells.Int J Oncol. 2012 Jun;40(6):1858-64. doi: 10.3892/ijo.2012.1407. Epub 2012 Mar 19. Int J Oncol. 2012. PMID: 22447278 Free PMC article.
-
High-dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer.Cochrane Database Syst Rev. 2016 May 20;2016(5):CD003139. doi: 10.1002/14651858.CD003139.pub3. Cochrane Database Syst Rev. 2016. PMID: 27200512 Free PMC article.
References
-
- Roche H, Viens P, Biron P, et al. High-dose chemotherapy for breast cancer: the French PEGASE experience. Cancer Control. 2003;10:42–47. - PubMed
-
- Nitz U, Mohrmann S, Fischer J, et al. Comparison of rapidly cycled tandem high dose chemotherapy plus peripheral-blood stem-cell support versus dose-dense conventional chemotherapy for adjuvant treatment of high-risk breast cancer: results of a multicentre phase III trial. Lancet. 2005;366:1935–1944. - PubMed
-
- Zander AR, Kroeger N, Schmoor C, et al. High-dose chemotherapy with autologous hematopoietic stem-cell support compared with standard-dose chemotherapy in breast cancer patients with 10 or more positive lymph nodes: first results of a randomized trial. J Clin Oncol. 2004;22:2273–2283. - PubMed
-
- Rodenhuis S, Bontenbal M, Beex LV, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for high risk breast cancer. N Engl J Med. 2003;349:7–16. - PubMed
-
- Leonard R, Lind M, Twelves C, et al. Conventional adjuvant chemotherapy versus single-cycle, autograft-supported, high-dose, late-intensification chemotherapy in high-risk breast cancer patients: a randomized trial. J Natl Cancer Inst. 2004;96:1076–1083. - PubMed

