A trans-membrane segment inside the ribosome exit tunnel triggers RAMP4 recruitment to the Sec61p translocase
- PMID: 19468070
- PMCID: PMC2711601
- DOI: 10.1083/jcb.200807066
A trans-membrane segment inside the ribosome exit tunnel triggers RAMP4 recruitment to the Sec61p translocase
Abstract
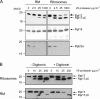
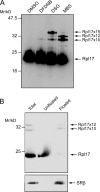
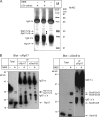
Membrane protein integration occurs predominantly at the endoplasmic reticulum and is mediated by the translocon, which is formed by the Sec61p complex. The translocon binds to the ribosome at the polypeptide exit site such that integration occurs in a cotranslational manner. Ribosomal protein Rpl17 is positioned such that it contacts both the ribosome exit tunnel and the surface of the ribosome near the exit site, where it is intimately associated with the translocon. The presence of a trans-membrane (TM) segment inside the ribosomal exit tunnel leads to the recruitment of RAMP4 to the translocon at a site adjacent to Rpl17. This suggests a signaling function for Rpl17 such that it can recognize a TM segment inside the ribosome and triggers rearrangements of the translocon, priming it for subsequent TM segment integration.
Figures





Comment in
-
The structural and functional coupling of two molecular machines, the ribosome and the translocon.J Cell Biol. 2009 Jun 1;185(5):765-7. doi: 10.1083/jcb.200902014. Epub 2009 May 25. J Cell Biol. 2009. PMID: 19468072 Free PMC article.
References
-
- Ban N., Nissen P., Hansen J., Moore P.B., Steitz T.A. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å.Science. 289:905–920 - PubMed
-
- Beckmann R., Spahn C.M.T., Eswar N., Helmers J., Penczek P.A., Sall A., Frank J., Blobel G. 2001. Architecture of the protein-conducting channel associated with the translating 80S ribosome.Cell. 107:361–372 - PubMed
-
- Besemer J., Harant H., Wang S., Oberhauser B., Marquardt K., Foster C.A., Schreiner E.P., de Vries J.E., Dascher-Nadel C., Lindley I.J. 2005. Selective inhibition of cotranslational translocation of vascular cell adhesion molecule 1.Nature. 436:290–293 - PubMed
-
- Bornemann T., Jockel J., Rodnina M.V., Wintermeyer W. 2008. Signal sequence-independent membrane targeting of ribosomes containing short nascent peptides within the exit tunnel.Nat. Struct. Mol. Biol. 15:494–499 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

