Rescue of skeletal muscle alpha-actin-null mice by cardiac (fetal) alpha-actin
- PMID: 19468071
- PMCID: PMC2711600
- DOI: 10.1083/jcb.200812132
Rescue of skeletal muscle alpha-actin-null mice by cardiac (fetal) alpha-actin
Abstract
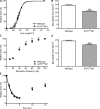
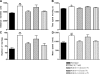
Skeletal muscle alpha-actin (ACTA1) is the major actin in postnatal skeletal muscle. Mutations of ACTA1 cause mostly fatal congenital myopathies. Cardiac alpha-actin (ACTC) is the major striated actin in adult heart and fetal skeletal muscle. It is unknown why ACTC and ACTA1 expression switch during development. We investigated whether ACTC can replace ACTA1 in postnatal skeletal muscle. Two ACTC transgenic mouse lines were crossed with Acta1 knockout mice (which all die by 9 d after birth). Offspring resulting from the cross with the high expressing line survive to old age, and their skeletal muscles show no gross pathological features. The mice are not impaired on grip strength, rotarod, or locomotor activity. These findings indicate that ACTC is sufficiently similar to ACTA1 to produce adequate function in postnatal skeletal muscle. This raises the prospect that ACTC reactivation might provide a therapy for ACTA1 diseases. In addition, the mouse model will allow analysis of the precise functional differences between ACTA1 and ACTC.
Figures





Similar articles
-
Skeletal and cardiac α-actin isoforms differently modulate myosin cross-bridge formation and myofibre force production.Hum Mol Genet. 2013 Nov 1;22(21):4398-404. doi: 10.1093/hmg/ddt289. Epub 2013 Jun 19. Hum Mol Genet. 2013. PMID: 23784376
-
Cardiac α-actin over-expression therapy in dominant ACTA1 disease.Hum Mol Genet. 2013 Oct 1;22(19):3987-97. doi: 10.1093/hmg/ddt252. Epub 2013 Jun 4. Hum Mol Genet. 2013. PMID: 23736297
-
Mouse models of dominant ACTA1 disease recapitulate human disease and provide insight into therapies.Brain. 2011 Apr;134(Pt 4):1101-15. doi: 10.1093/brain/awr004. Epub 2011 Feb 8. Brain. 2011. PMID: 21303860
-
Skeletal muscle α-actin diseases (actinopathies): pathology and mechanisms.Acta Neuropathol. 2013 Jan;125(1):19-32. doi: 10.1007/s00401-012-1019-z. Epub 2012 Jul 24. Acta Neuropathol. 2013. PMID: 22825594 Review.
-
Muscle disease caused by mutations in the skeletal muscle alpha-actin gene (ACTA1).Neuromuscul Disord. 2003 Sep;13(7-8):519-31. doi: 10.1016/s0960-8966(03)00101-9. Neuromuscul Disord. 2003. PMID: 12921789 Review.
Cited by
-
Congenital myopathies: an update.Curr Neurol Neurosci Rep. 2012 Apr;12(2):165-74. doi: 10.1007/s11910-012-0255-x. Curr Neurol Neurosci Rep. 2012. PMID: 22392505 Free PMC article. Review.
-
Differential roles of α-, β-, and γ-actin in axon growth and collateral branch formation in motoneurons.J Cell Biol. 2017 Mar 6;216(3):793-814. doi: 10.1083/jcb.201604117. Epub 2017 Feb 28. J Cell Biol. 2017. PMID: 28246119 Free PMC article.
-
Smooth muscle α actin is specifically required for the maintenance of lactation.Dev Biol. 2012 Mar 1;363(1):1-14. doi: 10.1016/j.ydbio.2011.11.002. Epub 2011 Nov 12. Dev Biol. 2012. PMID: 22123032 Free PMC article.
-
Absence of the Drosophila jump muscle actin Act79B is compensated by up-regulation of Act88F.Dev Dyn. 2018 Apr;247(4):642-649. doi: 10.1002/dvdy.24616. Epub 2018 Feb 6. Dev Dyn. 2018. PMID: 29318731 Free PMC article.
-
Comprehensive SHAP Values and Single-Cell Sequencing Technology Reveal Key Cell Clusters in Bovine Skeletal Muscle.Int J Mol Sci. 2025 Feb 26;26(5):2054. doi: 10.3390/ijms26052054. Int J Mol Sci. 2025. PMID: 40076676 Free PMC article.
References
-
- Brennan K.J., Hardeman E.C. 1993. Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice.J. Biol. Chem. 268:719–725 - PubMed
-
- Briguet A., Courdier-Fruh I., Foster M., Meier T., Magyar J.P. 2004. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse.Neuromuscul. Disord. 14:675–682 - PubMed
-
- Corbett M.A., Robinson C.S., Dunglison G.F., Yang N., Joya J.E., Stewart A.W., Schnell C., Gunning P.W., North K.N., Hardeman E.C. 2001. A mutation in alpha-tropomyosin(slow) affects muscle strength, maturation and hypertrophy in a mouse model for nemaline myopathy.Hum. Mol. Genet. 10:317–328 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

