CO2-induced ion and fluid transport in human retinal pigment epithelium
- PMID: 19468075
- PMCID: PMC2713148
- DOI: 10.1085/jgp.200810169
CO2-induced ion and fluid transport in human retinal pigment epithelium
Abstract
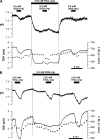
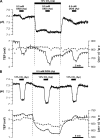
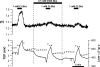
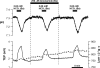
In the intact eye, the transition from light to dark alters pH, [Ca2+], and [K] in the subretinal space (SRS) separating the photoreceptor outer segments and the apical membrane of the retinal pigment epithelium (RPE). In addition to these changes, oxygen consumption in the retina increases with a concomitant release of CO2 and H2O into the SRS. The RPE maintains SRS pH and volume homeostasis by transporting these metabolic byproducts to the choroidal blood supply. In vitro, we mimicked the transition from light to dark by increasing apical bath CO2 from 5 to 13%; this maneuver decreased cell pH from 7.37 +/- 0.05 to 7.14 +/- 0.06 (n = 13). Our analysis of native and cultured fetal human RPE shows that the apical membrane is significantly more permeable (approximately 10-fold; n = 7) to CO2 than the basolateral membrane, perhaps due to its larger exposed surface area. The limited CO2 diffusion at the basolateral membrane promotes carbonic anhydrase-mediated HCO3 transport by a basolateral membrane Na/nHCO3 cotransporter. The activity of this transporter was increased by elevating apical bath CO2 and was reduced by dorzolamide. Increasing apical bath CO2 also increased intracellular Na from 15.7 +/- 3.3 to 24.0 +/- 5.3 mM (n = 6; P < 0.05) by increasing apical membrane Na uptake. The CO2-induced acidification also inhibited the basolateral membrane Cl/HCO3 exchanger and increased net steady-state fluid absorption from 2.8 +/- 1.6 to 6.7 +/- 2.3 microl x cm(-2) x hr(-1) (n = 5; P < 0.05). The present experiments show how the RPE can accommodate the increased retinal production of CO2 and H(2)O in the dark, thus preventing acidosis in the SRS. This homeostatic process would preserve the close anatomical relationship between photoreceptor outer segments and RPE in the dark and light, thus protecting the health of the photoreceptors.
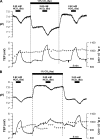
Figures








References
-
- Alm A., Bill A. 1987. Ocular circulation. Adler’s Physiology of the Eye. 8th ed Moses R.A., Hart W.M., Mosby, St. Louis, MO. 183–203
-
- Aronson P.S., Nee J., Suhm M.A. 1982. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles.Nature. 299:161–163 - PubMed
-
- Aronson P.S., Suhm M.A., Nee J. 1983. Interaction of external H+ with the Na+-H+ exchanger in renal microvillus membrane vesicles.J. Biol. Chem. 258:6767–6771 - PubMed
-
- Bevensee M.O., Schwiening C.J., Boron W.F. 1995. Use of BCECF and propidium iodide to assess membrane integrity of acutely isolated CA1 neurons from rat hippocampus.J. Neurosci. Methods. 58:61–75 - PubMed
-
- Bhosale P., Bernstein P.S. 2005. Quantitative measurement of 3′-oxolutein from human retina by normal-phase high-performance liquid chromatography coupled to atmospheric pressure chemical ionization mass spectrometry.Anal. Biochem. 345:296–301 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

