Proviral loads and clonal expansion of HTLV-1-infected cells following vertical transmission: a 10-year follow-up of children in Jamaica
- PMID: 19468234
- PMCID: PMC2790750
- DOI: 10.1159/000219384
Proviral loads and clonal expansion of HTLV-1-infected cells following vertical transmission: a 10-year follow-up of children in Jamaica
Abstract
Objective: Few studies have specifically examined proviral load (PVL) and clonal evolution of human T-lymphotropic virus type 1 (HTLV-1)-infected cells in vertically infected children.
Methods: Sequential samples (from ages 1 to 16 years) from 3 HTLV-1-infected children (cases A, B and C) in the Jamaica Mother Infant Cohort Study were analyzed for their PVL and clonal expansion of HTLV-1-infected cells in peripheral blood mononuclear cells (PBMCs) by inverse-long PCR.
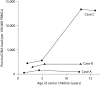
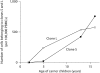
Results: The baseline PVL (per 100,000 PBMCs) of case A was 260 (at 1 year of age) and of case B it was 1,867 (at 3 years of age), and they remained constant for more than 10 years. Stochastic patterns of clonal expansion of HTLV-1-infected cells were predominately detected. In contrast, case C, who had lymphadenopathy, seborrheic dermatitis and hyperreflexia, showed an increase in PVL from 2,819 at 1.9 years to 13,358 at 13 years of age, and expansion of 2 dominant clones.
Conclusion: The clonal expansion of HTLV-1-infected cells is induced in early childhood after infection acquired from their mothers. Youths with high PVL and any signs and symptoms associated with HTLV-1 infection should be closely monitored.
Copyright 2009 S. Karger AG, Basel.
Figures




Similar articles
-
High HTLV-1 Proviral Load Predates and Predicts HTLV-1-Associated Disease: Literature Review and the London Experience.Pathogens. 2024 Jul 1;13(7):553. doi: 10.3390/pathogens13070553. Pathogens. 2024. PMID: 39057780 Free PMC article. Review.
-
Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: implication of high antiviral antibody titer and high proviral load in carrier mothers.Int J Cancer. 1999 Sep 9;82(6):832-6. doi: 10.1002/(sici)1097-0215(19990909)82:6<832::aid-ijc11>3.0.co;2-p. Int J Cancer. 1999. PMID: 10446450
-
Higher human T lymphotropic virus (HTLV) provirus load is associated with HTLV-I versus HTLV-II, with HTLV-II subtype A versus B, and with male sex and a history of blood transfusion.J Infect Dis. 2004 Aug 1;190(3):504-10. doi: 10.1086/422398. Epub 2004 Jul 6. J Infect Dis. 2004. PMID: 15243924
-
Use of synthetic oligonucleotides for determination of HTLV-1 proviral load by real-time PCR: a helpful alternative approach in the clinical management.J Appl Microbiol. 2020 Sep;129(3):768-774. doi: 10.1111/jam.14646. Epub 2020 Apr 8. J Appl Microbiol. 2020. PMID: 32202037
-
Evaluation of HTLV-1 HBZ and proviral load, together with host IFN λ3, in pathogenesis of HAM/TSP.J Med Virol. 2017 Jun;89(6):1102-1107. doi: 10.1002/jmv.24721. Epub 2016 Nov 10. J Med Virol. 2017. PMID: 27787900
Cited by
-
Clinical and Public Health Implications of Human T-Lymphotropic Virus Type 1 Infection.Clin Microbiol Rev. 2022 Apr 20;35(2):e0007821. doi: 10.1128/cmr.00078-21. Epub 2022 Feb 23. Clin Microbiol Rev. 2022. PMID: 35195446 Free PMC article. Review.
-
NF-κB-Induced R-Loops and Genomic Instability in HTLV-1-Infected and Adult T-Cell Leukemia Cells.Viruses. 2022 Apr 23;14(5):877. doi: 10.3390/v14050877. Viruses. 2022. PMID: 35632619 Free PMC article. Review.
-
HTLV-1 Infection and Adult T-Cell Leukemia/Lymphoma-A Tale of Two Proteins: Tax and HBZ.Viruses. 2016 Jun 16;8(6):161. doi: 10.3390/v8060161. Viruses. 2016. PMID: 27322308 Free PMC article. Review.
-
Immunological profile of HTLV-1-infected patients associated with infectious or autoimmune dermatological disorders.PLoS Negl Trop Dis. 2013 Jul 25;7(7):e2328. doi: 10.1371/journal.pntd.0002328. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23936564 Free PMC article.
-
Dynamics of perinatal bovine leukemia virus infection.BMC Vet Res. 2014 Apr 4;10:82. doi: 10.1186/1746-6148-10-82. BMC Vet Res. 2014. PMID: 24708791 Free PMC article.
References
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. - PubMed
-
- Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–1032. - PubMed
-
- Arisawa K, Soda M, Endo S, Kurokawa K, Katamine S, Shimokawa I, Koba T, Takahashi T, Saito H, Doi H, Shirahama S. Evaluation of adult T-cell leukemia/lymphoma incidence and its impact on non-Hodgkin lymphoma incidence in southwestern Japan. Int J Cancer. 2000;85:319–324. - PubMed
-
- Yamaguchi K, Watanabe T. Human T lymphotropic virus type-I and adult T-cell leukemia in Japan. Int J Hematol. 2002;76:240–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

