Intrinsic thermal sensing controls proteolysis of Yersinia virulence regulator RovA
- PMID: 19468295
- PMCID: PMC2676509
- DOI: 10.1371/journal.ppat.1000435
Intrinsic thermal sensing controls proteolysis of Yersinia virulence regulator RovA
Abstract
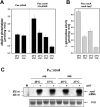
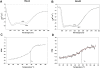
Pathogens, which alternate between environmental reservoirs and a mammalian host, frequently use thermal sensing devices to adjust virulence gene expression. Here, we identify the Yersinia virulence regulator RovA as a protein thermometer. Thermal shifts encountered upon host entry lead to a reversible conformational change of the autoactivator, which reduces its DNA-binding functions and renders it more susceptible for proteolysis. Cooperative binding of RovA to its target promoters is significantly reduced at 37 degrees C, indicating that temperature control of rovA transcription is primarily based on the autoregulatory loop. Thermally induced reduction of DNA-binding is accompanied by an enhanced degradation of RovA, primarily by the Lon protease. This process is also subject to growth phase control. Studies with modified/chimeric RovA proteins indicate that amino acid residues in the vicinity of the central DNA-binding domain are important for proteolytic susceptibility. Our results establish RovA as an intrinsic temperature-sensing protein in which thermally induced conformational changes interfere with DNA-binding capacity, and secondarily render RovA susceptible to proteolytic degradation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


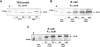

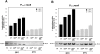



Similar articles
-
Structural basis for intrinsic thermosensing by the master virulence regulator RovA of Yersinia.J Biol Chem. 2012 Oct 19;287(43):35796-803. doi: 10.1074/jbc.M112.379156. Epub 2012 Aug 30. J Biol Chem. 2012. PMID: 22936808 Free PMC article.
-
RovM, a novel LysR-type regulator of the virulence activator gene rovA, controls cell invasion, virulence and motility of Yersinia pseudotuberculosis.Mol Microbiol. 2006 Dec;62(5):1469-83. doi: 10.1111/j.1365-2958.2006.05458.x. Epub 2006 Oct 27. Mol Microbiol. 2006. PMID: 17074075
-
Analysis of RovA, a transcriptional regulator of Yersinia pseudotuberculosis virulence that acts through antirepression and direct transcriptional activation.J Biol Chem. 2005 Dec 23;280(51):42423-32. doi: 10.1074/jbc.M504464200. Epub 2005 Oct 27. J Biol Chem. 2005. PMID: 16257976
-
Regulatory elements implicated in the environmental control of invasin expression in enteropathogenic Yersinia.Adv Exp Med Biol. 2007;603:156-66. doi: 10.1007/978-0-387-72124-8_13. Adv Exp Med Biol. 2007. PMID: 17966412 Review.
-
Invasin and beyond: regulation of Yersinia virulence by RovA.Trends Microbiol. 2004 Jun;12(6):296-300. doi: 10.1016/j.tim.2004.04.006. Trends Microbiol. 2004. PMID: 15165608 Review.
Cited by
-
Structural basis for intrinsic thermosensing by the master virulence regulator RovA of Yersinia.J Biol Chem. 2012 Oct 19;287(43):35796-803. doi: 10.1074/jbc.M112.379156. Epub 2012 Aug 30. J Biol Chem. 2012. PMID: 22936808 Free PMC article.
-
Unique cell adhesion and invasion properties of Yersinia enterocolitica O:3, the most frequent cause of human Yersiniosis.PLoS Pathog. 2011 Jul;7(7):e1002117. doi: 10.1371/journal.ppat.1002117. Epub 2011 Jul 7. PLoS Pathog. 2011. PMID: 21750675 Free PMC article.
-
The Protease Locus of Francisella tularensis LVS Is Required for Stress Tolerance and Infection in the Mammalian Host.Infect Immun. 2016 Apr 22;84(5):1387-1402. doi: 10.1128/IAI.00076-16. Print 2016 May. Infect Immun. 2016. PMID: 26902724 Free PMC article.
-
Molecular basis of Yersinia enterocolitica temperature-dependent resistance to antimicrobial peptides.J Bacteriol. 2012 Jun;194(12):3173-88. doi: 10.1128/JB.00308-12. Epub 2012 Apr 13. J Bacteriol. 2012. PMID: 22505678 Free PMC article.
-
The LonA Protease Regulates Biofilm Formation, Motility, Virulence, and the Type VI Secretion System in Vibrio cholerae.J Bacteriol. 2016 Jan 11;198(6):973-85. doi: 10.1128/JB.00741-15. J Bacteriol. 2016. PMID: 26755629 Free PMC article.
References
-
- Konkel ME, Tilly K. Temperature-regulated expression of bacterial virulence genes. Microbes Infect. 2000;2:157–166. - PubMed
-
- Schumann W. Thermosensors in eubacteria: role and evolution. J Biosci. 2007;32:549–557. - PubMed
-
- Straley SC, Perry RD. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 1995;3:310–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

