Neo-lymphoid aggregates in the adult liver can initiate potent cell-mediated immunity
- PMID: 19468301
- PMCID: PMC2680335
- DOI: 10.1371/journal.pbio.1000109
Neo-lymphoid aggregates in the adult liver can initiate potent cell-mediated immunity
Abstract
Subcutaneous immunization delivers antigen (Ag) to local Ag-presenting cells that subsequently migrate into draining lymph nodes (LNs). There, they initiate the activation and expansion of lymphocytes specific for their cognate Ag. In mammals, the structural environment of secondary lymphoid tissues (SLTs) is considered essential for the initiation of adaptive immunity. Nevertheless, cold-blooded vertebrates can initiate potent systemic immune responses even though they lack conventional SLTs. The emergence of lymph nodes provided mammals with drastically improved affinity maturation of B cells. Here, we combine the use of different strains of alymphoplastic mice and T cell migration mutants with an experimental paradigm in which the site of Ag delivery is distant from the site of priming and inflammation. We demonstrate that in mammals, SLTs serve primarily B cell priming and affinity maturation, whereas the induction of T cell-driven immune responses can occur outside of SLTs. We found that mice lacking conventional SLTs generate productive systemic CD4- as well as CD8-mediated responses, even under conditions in which draining LNs are considered compulsory for the initiation of adaptive immunity. We describe an alternative pathway for the induction of cell-mediated immunity (CMI), in which Ag-presenting cells sample Ag and migrate into the liver where they induce neo-lymphoid aggregates. These structures are insufficient to support antibody affinity maturation and class switching, but provide a novel surrogate environment for the initiation of CMI.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

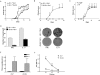


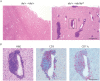

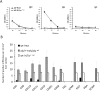
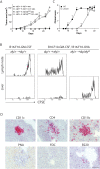
Comment in
-
Liver is T cells' ace in the hole.PLoS Biol. 2009 May 26;7(5):e1000113. doi: 10.1371/journal.pbio.1000113. PLoS Biol. 2009. PMID: 20076743 Free PMC article. No abstract available.
References
-
- Zinkernagel RM, Ehl S, Aichele P, Oehen S, Kundig T, et al. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev. 1997;156:199–209. - PubMed
-
- Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. - PubMed
-
- Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–1064. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

