Conservation of arthropod midline netrin accumulation revealed with a cross-reactive antibody provides evidence for midline cell homology
- PMID: 19469853
- PMCID: PMC2749990
- DOI: 10.1111/j.1525-142X.2009.00328.x
Conservation of arthropod midline netrin accumulation revealed with a cross-reactive antibody provides evidence for midline cell homology
Abstract
Although many similarities in arthropod CNS development exist, differences in axonogenesis and the formation of midline cells, which regulate axon growth, have been observed. For example, axon growth patterns in the ventral nerve cord of Artemia franciscana differ from that of Drosophila melanogaster. Despite such differences, conserved molecular marker expression at the midline of several arthropod species indicates that midline cells may be homologous in distantly related arthropods. However, data from additional species are needed to test this hypothesis. In this investigation, nerve cord formation and the putative homology of midline cells were examined in distantly related arthropods, including: long- and short-germ insects (D. melanogaster, Aedes aeygypti, and Tribolium castaneum), branchiopod crustaceans (A. franciscana and Triops longicauditus), and malacostracan crustaceans (Porcellio laevis and Parhyale hawaiensis). These comparative analyses were aided by a cross-reactive antibody generated against the Netrin (Net) protein, a midline cell marker and regulator of axonogenesis. The mechanism of nerve cord formation observed in Artemia is found in Triops, another branchiopod, but is not found in the other arthropods examined. Despite divergent mechanisms of midline cell formation and nerve cord development, Net accumulation is detected in a well-conserved subset of midline cells in branchiopod crustaceans, malacostracan crustaceans, and insects. Notably, the Net accumulation pattern is also conserved at the midline of the amphipod P. hawaiensis, which undergoes split germ-band development. Conserved Net accumulation patterns indicate that arthropod midline cells are homologous, and that Nets function to regulate commissure formation during CNS development of Tetraconata.
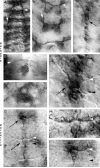
Figures


References
-
- Averof M, Patel NH. Crustacean appendage evolution associated with changes in Hox gene expression. Nature. 1997;388:682–686. - PubMed
-
- Blanchard CE. Pioneer neurons and the early development of the nervous system in Artemia. In: Sorgeloss P, Bengtson DA, Declair W, Jaspers E, editors. Artemia Research and its Applications. I. Universa Press; Wetteren, Belgium: 1987. pp. 5–32.
-
- Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat. Neurosci. 2006;9:188–194. - PubMed
-
- Browne WE, Price AL, Gerberding M, Patel NH. Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis. Genesis. 2005;42:124–149. - PubMed
-
- Browne WE, Schmid BGM, Wimmer EA, Martindale MQ. Expression of otd orthologs in the amphipod crustacean, Parhyale hawaiensis. Dev. Genes Evol. 2006;216:581–595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

