Pharmacokinetics, tissue distribution and mass balance of radiolabeled dihydroartemisinin in male rats
- PMID: 19470172
- PMCID: PMC2694832
- DOI: 10.1186/1475-2875-8-112
Pharmacokinetics, tissue distribution and mass balance of radiolabeled dihydroartemisinin in male rats
Abstract
Background: Dihydroartemisinin (DHA), a powerful anti-malarial drug, has been used as monotherapy and artemisinin-based combination therapy (ACT) for more than decades. So far, however, the tissue distribution and metabolic profile of DHA data are not available from animal and humans.
Methods: Pharmacokinetics, tissue distribution, mass balance, and elimination of [14C] DHA have been studieded in rats following a single intravenous administration. Protein binding was performed with rat and human plasma. Drug concentrations were obtained up to 192 hr from measurements of total radioactivity and drug concentration to determine the contribution by the parent and metabolites to the total dose of drug injected from whole blood, plasma, urine and faecal samples.
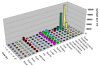
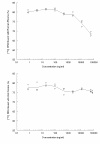
Results: Drug was widely distributed after 1 hr and rapidly declined at 24 hr in all tissues except spleen until 96 hrs. Only 0.81% of the total radioactivity was detected in rat brain tissue. DHA revealed a high binding capacity with both rat and human plasma proteins (76-82%). The concentration of total radioactivity in the plasma fraction was less than 25% of that in blood total. Metabolism of DHA was observed with high excretion via bile into intestines and approximately 89-95% dose of all conjugations were accounted for in blood, urine and faeces. However, the majority of elimination of [14C] DHA was through urinary excretion (52% dose). The mean terminal half-lives of plasma and blood radioactivity (75.57-122.13 h) were significantly prolonged compared with that of unchanged DHA (1.03 h).
Conclusion: In rat brain, the total concentration of [14C] was 2-fold higher than that in plasma, indicating the radioactivity could easily penetrate the brain-blood barrier. Total radioactivity distributed in RBC was about three- to four-fold higher than that in plasma, suggesting that the powerful anti-malarial potency of DHA in the treatment of blood stage malaria may relate to the high RBC binding. Biliary excretion and multiple concentration peaks of DHA have been demonstrated with high urinary excretion due to a most likely drug re-absorption in the intestines (enterohepatic circulation). The long lasting metabolites of DHA (> 192 hr) in the rats may be also related to the enterohepatic circulation.
Figures


Similar articles
-
The distribution pattern of intravenous [(14)C] artesunate in rat tissues by quantitative whole-body autoradiography and tissue dissection techniques.J Pharm Biomed Anal. 2008 Nov 4;48(3):876-84. doi: 10.1016/j.jpba.2008.07.015. Epub 2008 Jul 30. J Pharm Biomed Anal. 2008. PMID: 18762400
-
The evaluation of radiolabeled artesunate on tissue distribution in rats and protein binding in humans.Am J Trop Med Hyg. 2006 Nov;75(5):817-26. Am J Trop Med Hyg. 2006. PMID: 17123971
-
Toxicokinetics and hydrolysis of artelinate and artesunate in malaria-infected rats.Int J Toxicol. 2005 Jul-Aug;24(4):241-50. doi: 10.1080/10915810591007201. Int J Toxicol. 2005. PMID: 16126618
-
Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration.Malar J. 2011 Sep 13;10:263. doi: 10.1186/1475-2875-10-263. Malar J. 2011. PMID: 21914160 Free PMC article. Review.
-
Absorption, distribution, metabolism and excretion of glucosamine sulfate. A review.Arzneimittelforschung. 2001 Sep;51(9):699-725. doi: 10.1055/s-0031-1300105. Arzneimittelforschung. 2001. PMID: 11642003 Review.
Cited by
-
The effect of UGTs polymorphism on the auto-induction phase II metabolism-mediated pharmacokinetics of dihydroartemisinin in healthy Chinese subjects after oral administration of a fixed combination of dihydroartemisinin-piperaquine.Malar J. 2014 Dec 4;13:478. doi: 10.1186/1475-2875-13-478. Malar J. 2014. PMID: 25476790 Free PMC article.
-
Interspecies allometric scaling of antimalarial drugs and potential application to pediatric dosing.Antimicrob Agents Chemother. 2014 Oct;58(10):6068-78. doi: 10.1128/AAC.02538-14. Epub 2014 Aug 4. Antimicrob Agents Chemother. 2014. PMID: 25092696 Free PMC article.
-
Application of Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung and Trachea Exposure of Pyronaridine and Artesunate in Hamsters.Pharmaceutics. 2023 Mar 3;15(3):838. doi: 10.3390/pharmaceutics15030838. Pharmaceutics. 2023. PMID: 36986698 Free PMC article.
-
An investigation of the auto-induction of and gender-related variability in the pharmacokinetics of dihydroartemisinin in the rat.Malar J. 2012 Nov 21;11:379. doi: 10.1186/1475-2875-11-379. Malar J. 2012. PMID: 23171067 Free PMC article.
-
Predicting the Disposition of the Antimalarial Drug Artesunate and Its Active Metabolite Dihydroartemisinin Using Physiologically Based Pharmacokinetic Modeling.Antimicrob Agents Chemother. 2021 Feb 17;65(3):e02280-20. doi: 10.1128/AAC.02280-20. Print 2021 Feb 17. Antimicrob Agents Chemother. 2021. PMID: 33361307 Free PMC article.
References
-
- Li Q, Brewer TG, Peggins JO. Anorectic toxicity of dihydroartemisinin, arteether and artemether in rats following multiple intramuscular doses. International J Toxicol. 1998;17:663–676. doi: 10.1080/109158198225900. - DOI
-
- Li Q, Mog SR, Si YZ, Kyle DE, Gettayacamin M, Milhous WK. Neurotoxicity and efficacy of arteether related to its exposure times and exposure levels in rodents. Am J Trop Med Hyg. 2002;66:516–525. - PubMed
-
- McLean WG, Ward SA. In vitro neurotoxicity of artemisinin derivatives. Med Trop (Mars) . 1998;58:28–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

