Insights into the evolution of sialic acid catabolism among bacteria
- PMID: 19470179
- PMCID: PMC2693436
- DOI: 10.1186/1471-2148-9-118
Insights into the evolution of sialic acid catabolism among bacteria
Abstract
Background: Sialic acids comprise a family of nine-carbon amino sugars that are prevalent in mucus rich environments. Sialic acids from the human host are used by a number of pathogens as an energy source. Here we explore the evolution of the genes involved in the catabolism of sialic acid.
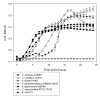
Results: The cluster of genes encoding the enzymes N-acetylneuraminate lyase (NanA), epimerase (NanE), and kinase (NanK), necessary for the catabolism of sialic acid (the Nan cluster), are confined 46 bacterial species, 42 of which colonize mammals, 33 as pathogens and 9 as gut commensals. We found a putative sialic acid transporter associated with the Nan cluster in most species. We reconstructed the phylogenetic history of the NanA, NanE, and NanK proteins from the 46 species and compared them to the species tree based on 16S rRNA. Within the NanA phylogeny, Gram-negative and Gram-positive bacteria do not form distinct clades. NanA from Yersinia and Vibrio species was most closely related to the NanA clade from eukaryotes. To examine this further, we reconstructed the phylogeny of all NanA homologues in the databases. In this analysis of 83 NanA sequences, Bacteroidetes, a human commensal group formed a distinct clade with Verrucomicrobia, and branched with the Eukaryotes and the Yersinia/Vibrio clades. We speculate that pathogens such as V. cholerae may have acquired NanA from a commensal aiding their colonization of the human gut. Both the NanE and NanK phylogenies more closely represented the species tree but numerous incidences of incongruence are noted. We confirmed the predicted function of the sialic acid catabolism cluster in members the major intestinal pathogens Salmonella enterica, Vibrio cholerae, V. vulnificus, Yersinia enterocolitica and Y. pestis.
Conclusion: The Nan cluster among bacteria is confined to human pathogens and commensals conferring them the ability to utilize a ubiquitous carbon source in mucus rich surfaces of the human body. The Nan region shows a mosaic evolution with NanA from Bacteroidetes, Vibrio and Yersinia branching closely together with NanA from eukaryotes.
Figures





References
-
- Warren L. Bound Carbohydrates in Nature. Cambridge University press; 1994.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

