Stem cells and liver regeneration
- PMID: 19470389
- PMCID: PMC3136245
- DOI: 10.1053/j.gastro.2009.05.044
Stem cells and liver regeneration
Abstract
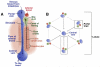
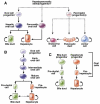
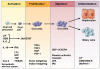
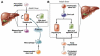
One of the defining features of the liver is the capacity to maintain a constant size despite injury. Although the precise molecular signals involved in the maintenance of liver size are not completely known, it is clear that the liver delicately balances regeneration with overgrowth. Mammals, for example, can survive surgical removal of up to 75% of the total liver mass. Within 1 week after liver resection, the total number of liver cells is restored. Moreover, liver overgrowth can be induced by a variety of signals, including hepatocyte growth factor or peroxisome proliferators; the liver quickly returns to its normal size when the proliferative signal is removed. The extent to which liver stem cells mediate liver regeneration has been hotly debated. One of the primary reasons for this controversy is the use of multiple definitions for the hepatic stem cell. Definitions for the liver stem cell include the following: (1) cells responsible for normal tissue turnover, (2) cells that give rise to regeneration after partial hepatectomy, (3) cells responsible for progenitor-dependent regeneration, (4) cells that produce hepatocyte and bile duct epithelial phenotypes in vitro, and (5) transplantable liver-repopulating cells. This review will consider liver stem cells in the context of each definition.
Figures
References
-
- Desmet VJ. Organizational principles. In: Arias IM, editor. The liver—biology and pathobiology. Raven; New York, NY: 1994. pp. 3–14.
-
- Mall FP. A study of the structural unit of the liver. Am J Anat. 1906;5:227–308.
-
- Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970;31:125–150. - PubMed
-
- Jungermann K, Kietzmann T. Zonation of parenchymal and non-parenchymal metabolism in liver. Annu Rev Nutr. 1996;16:179–203. - PubMed
-
- Moorman AF, Vermeulen JL, Charles R, et al. Localization of ammonia-metabolizing enzymes in human liver: ontogenesis of heterogeneity. Hepatology. 1989;9:367–372. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

