Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome
- PMID: 19470430
- PMCID: PMC2759838
- DOI: 10.1194/jlr.P900032-JLR200
Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome
Abstract
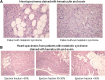
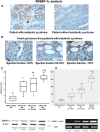
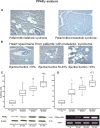
We evaluated the role of sterol-regulatory element binding protein (SREBP)-1c/peroxisome proliferator activated receptor-gamma (PPARgamma) pathway on heart lipotoxicity in patients with metabolic syndrome (MS) and aortic stenosis (AS). Echocardiographic parameters of heart function and structural alterations of LV specimens were studied in patients with (n = 56) and without (n = 61) MS undergoing aortic valve replacement. Tissues were stained with hematoxylin-eosin (H and E) and oil red O for evidence of intramyocyte lipid accumulation. The specimens were also analyzed with PCR, Western blot, and immunohistochemical analysis for SREBP-1c and PPARgamma. Ejection fraction (EF) was lower in MS compared with patients without MS (P < 0.001); no difference was found in aortic orifice surface among the groups. H and E and oil red O staining of specimens from MS patients revealed several myocytes with intracellular accumulation of lipid, whereas these alterations were not detected in biopsies from patients without MS. Patients without MS have low levels and weak immunostaining of SREBP-1c and PPARgamma in heart specimens. In contrast, strong immunostaining and higher levels of SREBP-1c and PPARgamma were seen in biopsies from the MS patients. Moreover, we evidenced a significative correlation between both SREBP-1c and PPARgamma and EF and intramyocyte lipid accumulation (P < 0.001). SREBP-1c may contribute to heart dysfunction by promoting lipid accumulation within myocytes in MS patients with AS; SREBP-1c may do it by increasing the levels of PPARgamma protein.
Figures


Comment in
-
Myocardial lipid accumulation and lipotoxicity in heart failure.J Lipid Res. 2009 Nov;50(11):2137-8. doi: 10.1194/jlr.R001115. Epub 2009 Aug 17. J Lipid Res. 2009. PMID: 19687505 Free PMC article. No abstract available.
References
-
- Burchfiel C. M., Skelton T. N., Andrew M. E., Garrison R. J., Arnett D. K., Jones D. W., Taylor H. A., Jr 2005. Metabolic syndrome and echocardiographic left ventricular mass in blacks: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 112: 819–827. - PubMed
-
- McGavock J. M., Victor R. G., Unger R. H., Szczepaniak L. S. 2006. Adiposity of the heart, revisited. Ann. Intern. Med. 144: 517–524. - PubMed
-
- McGavock J. M., Lingvay I., Zib I., Tillery T., Salas N., Unger R., Levine B. D., Raskin P., Victor R. G., Szczepaniak L. S. 2007. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 116: 1170–1175. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

