Molecular basis and phenotype of methicillin resistance in Staphylococcus aureus and insights into new beta-lactams that meet the challenge
- PMID: 19470504
- PMCID: PMC2764218
- DOI: 10.1128/AAC.00084-09
Molecular basis and phenotype of methicillin resistance in Staphylococcus aureus and insights into new beta-lactams that meet the challenge
Figures


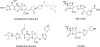
References
-
- Abbanat, D., B. Morrow, and K. Bush. 2008. New agents in development for the treatment of bacterial infections. Curr. Opin. Pharmacol. 8:582-592. - PubMed
-
- Aksoy, D. Y., and S. Unal. 2008. New antimicrobial agents for the treatment of gram-positive bacterial infections. Clin. Microbiol. Infect. 14:411-420. - PubMed
-
- Anderson, S. D., and J. G. Gums. 2008. Ceftobiprole: an extended-spectrum anti-methicillin-resistant Staphylococcus aureus cephalosporin. Ann. Pharmacother. 42:806-816. - PubMed
-
- Andes, D., and W. Craig. 2008. In vivo pharmacodynamic activity of carbapenem ME1036 in a murine thigh infection model, poster A-032. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents. Chemother. (ICAAC)/Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical

