A novel genetic mechanism regulates dorsolateral hinge-point formation during zebrafish cranial neurulation
- PMID: 19470582
- PMCID: PMC2723160
- DOI: 10.1242/jcs.043471
A novel genetic mechanism regulates dorsolateral hinge-point formation during zebrafish cranial neurulation
Abstract
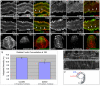
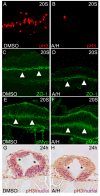
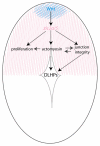
During neurulation, vertebrate embryos form a neural tube (NT), the rudiment of the central nervous system. In mammals and birds, a key step in cranial NT morphogenesis is dorsolateral hinge-point (DLHP) bending, which requires an apical actomyosin network. The mechanism of DLHP formation is poorly understood, although several essential genes have been identified, among them Zic2, which encodes a zinc-finger transcription factor. We found that DLHP formation in the zebrafish midbrain also requires actomyosin and Zic function. Given this conservation, we used the zebrafish to study how genes encoding Zic proteins regulate DLHP formation. We demonstrate that the ventral zic2a expression border predicts DLHP position. Using morpholino (MO) knockdown, we show zic2a and zic5 are required for apical F-actin and active myosin II localization and junction integrity. Furthermore, myosin II activity can function upstream of junction integrity during DLHP formation, and canonical Wnt signaling, an activator of zic gene transcription, is necessary for apical active myosin II localization, junction integrity and DLHP formation. We conclude that zic genes act downstream of Wnt signaling to control cytoskeletal organization, and possibly adhesion, during neurulation. This study identifies zic2a and zic5 as crucial players in the genetic network linking patterned gene expression to morphogenetic changes during neurulation, and strengthens the utility of the zebrafish midbrain as a NT morphogenesis model.
Figures







References
-
- Aaku-Saraste, E., Hellwig, A. and Huttner, W. B. (1996). Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure-remodeling of the neuroepithelium prior to neurogenesis. Dev. Biol. 180, 664-679. - PubMed
-
- Aruga, J. (2004). The role of Zic genes in neural development. Mol. Cell Neurosci. 26, 205-221. - PubMed
-
- Aruga, J., Nagai, T., Tokuyama, T., Hayashizaki, Y., Okazaki, Y., Chapman, V. M. and Mikoshiba, K. (1996). The mouse zic gene family: homologues of the Drosophila pair-rule gene odd-paired. J. Biol. Chem. 271, 1043-1047. - PubMed
-
- Bertet, C., Sulak, L. and Lecuit, T. (2004). Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667-671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

