Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement
- PMID: 19470590
- PMCID: PMC2700541
- DOI: 10.1105/tpc.108.064279
Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement
Abstract
Remorins (REMs) are proteins of unknown function specific to vascular plants. We have used imaging and biochemical approaches and in situ labeling to demonstrate that REM clusters at plasmodesmata and in approximately 70-nm membrane domains, similar to lipid rafts, in the cytosolic leaflet of the plasma membrane. From a manipulation of REM levels in transgenic tomato (Solanum lycopersicum) plants, we show that Potato virus X (PVX) movement is inversely related to REM accumulation. We show that REM can interact physically with the movement protein TRIPLE GENE BLOCK PROTEIN1 from PVX. Based on the localization of REM and its impact on virus macromolecular trafficking, we discuss the potential for lipid rafts to act as functional components in plasmodesmata and the plasma membrane.
Figures



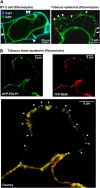
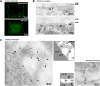



Comment in
-
Membrane rafts and virus movement in plant cells.Plant Cell. 2009 May;21(5):1326. doi: 10.1105/tpc.109.210511. Epub 2009 May 26. Plant Cell. 2009. PMID: 19470586 Free PMC article. No abstract available.
References
-
- Bariola, P.A., Retelska, D., Stasiak, A., Kammerer, R.A., Fleming, A., Hijri, M., Frank, S., and Farmer, E.E. (2004). Remorins form a novel family of coiled coil-forming oligomeric and filamentous proteins associated with apical, vascular and embryonic tissues in plants. Plant Mol. Biol. 55 579–594. - PubMed
-
- Bhat, R.A., and Panstruga, R. (2005). Lipid rafts in plants. Planta 223 5–19. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

