Cinacalcet reduces serum calcium concentrations in patients with intractable primary hyperparathyroidism
- PMID: 19470620
- PMCID: PMC3214593
- DOI: 10.1210/jc.2008-2640
Cinacalcet reduces serum calcium concentrations in patients with intractable primary hyperparathyroidism
Abstract
Context: Patients with persistent primary hyperparathyroidism (PHPT) after parathyroidectomy or with contraindications to parathyroidectomy often require chronic treatment for hypercalcemia.
Objective: The objective of the study was to assess the ability of the calcimimetic, cinacalcet, to reduce serum calcium in patients with intractable PHPT.
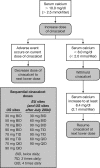
Design: This was an open-label, single-arm study comprising a titration phase of variable duration (2-16 wk) and a maintenance phase of up to 136 wk.
Setting: The study was conducted at 23 centers in Europe, the United States, and Canada.
Patients: The study included 17 patients with intractable PHPT and serum calcium greater than 12.5 mg/dl (3.1 mmol/liter).
Intervention: During the titration phase, cinacalcet dosages were titrated every 2 wk (30 mg twice daily to 90 mg four times daily) for 16 wk until serum calcium was 10 mg/dl or less (2.5 mmol/liter). If serum calcium increased during the maintenance phase, additional increases in the cinacalcet dose were permitted.
Main outcome measure: The primary end point was the proportion of patients experiencing a reduction in serum calcium of 1 mg/dl or greater (0.25 mmol/liter) at the end of the titration phase.
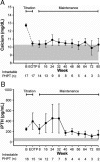
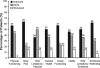
Results: Mean +/- sd baseline serum calcium was 12.7 +/- 0.8 mg/dl (3.2 +/- 0.2 mmol/liter). At the end of titration, a 1 mg/dl or greater reduction in serum calcium was achieved in 15 patients (88%). Fifteen patients (88%) experienced treatment-related adverse events, none of which were serious. The most common adverse events were nausea, vomiting, and paresthesias.
Conclusions: In patients with intractable PHPT, cinacalcet reduces serum calcium, is generally well tolerated, and has the potential to fulfill an unmet medical need.
Trial registration: ClinicalTrials.gov NCT00037518.
Figures
References
-
- AACE/AAES Task Force on Primary Hyperparathyroidism 2005 The American Association of Clinical Endocrinologists and the American Association of Endocrine Surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract 11:49–54 - PubMed
-
- Heath 3rd H 1991 Clinical spectrum of primary hyperparathyroidism: evolution with changes in medical practice and technology. J Bone Miner Res 6(Suppl 2):S63–S70; discussion S83–S84 - PubMed
-
- Boggs JE, Irvin 3rd GL, Carneiro DM, Molinari AS 1999 The evolution of parathyroidectomy failures. Surgery 126:998–1002; discussion 1002–1003 - PubMed
-
- Hasse C, Sitter H, Brune M, Wollenteit I, Nies C, Rothmund M 2002 Quality of life and patient satisfaction after reoperation for primary hyperparathyroidism: analysis of long-term results. World J Surg 26:1029–1036 - PubMed
-
- Hedback G, Oden A 2003 Recurrence of hyperparathyroidism: a long-term follow-up after surgery for primary hyperparathyroidism. Eur J Endocrinol 148:413–421 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

