Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase
- PMID: 19470642
- PMCID: PMC2722301
- DOI: 10.1073/pnas.0900689106
Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase
Abstract
The entry of carbon from sucrose into cellular metabolism in plants can potentially be catalyzed by either sucrose synthase (SUS) or invertase (INV). These 2 routes have different implications for cellular metabolism in general and for the production of key metabolites, including the cell-wall precursor UDPglucose. To examine the importance of these 2 routes of sucrose catabolism in Arabidopsis thaliana (L.), we generated mutant plants that lack 4 of the 6 isoforms of SUS. These mutants (sus1/sus2/sus3/sus4 mutants) lack SUS activity in all cell types except the phloem. Surprisingly, the mutant plants are normal with respect to starch and sugar content, seed weight and lipid content, cellulose content, and cell-wall structure. Plants lacking the remaining 2 isoforms of SUS (sus5/sus6 mutants), which are expressed specifically in the phloem, have reduced amounts of callose in the sieve plates of the sieve elements. To discover whether sucrose catabolism in Arabidopsis requires INVs rather than SUSs, we further generated plants deficient in 2 closely related isoforms of neutral INV predicted to be the main cytosolic forms in the root (cinv1/cinv2 mutants). The mutant plants have severely reduced growth rates. We discuss the implications of these findings for our understanding of carbon supply to the nonphotosynthetic cells of plants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
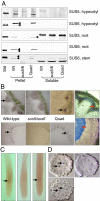
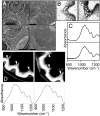
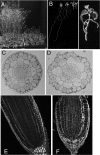
References
-
- Zrenner R, Salanoubat M, Willmitzer L, Sonnewald U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.) Plant J. 1995;7:97–107. - PubMed
-
- Chourey PS, Taliercio EW, Carlson SJ, Ruan YL. Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol Gen Genet. 1998;259:88–96. - PubMed
-
- Craig J, et al. Mutations at the rug4 locus alter the carbon and nitrogen metabolism of pea plants through an effect on sucrose synthase. Plant J. 1999;17:353–362.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

