Mutational and functional analysis of Large in a novel CHO glycosylation mutant
- PMID: 19470663
- PMCID: PMC2720279
- DOI: 10.1093/glycob/cwp074
Mutational and functional analysis of Large in a novel CHO glycosylation mutant
Abstract
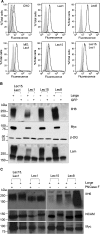
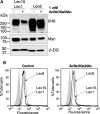
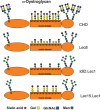
Inactivating mutations of Large reduce the functional glycosylation of alpha-dystroglycan (alpha-DG) and lead to muscular dystrophy in mouse and humans. The N-terminal domain of Large is most similar to UDP-glucose glucosyltransferases (UGGT), and the C-terminal domain is related to the human i blood group transferase beta1,3GlcNAcT-1. The amino acids at conserved motifs DQD+1 and DQD+3 in the UGGT domain are necessary for mammalian UGGT activity. When the corresponding residues were mutated to Ala in mouse Large, alpha-DG was not functionally glycosylated. A similar result was obtained when a DXD motif in the beta1,3GlcNAcT-1 domain was mutated to AIA. Therefore, the first putative glycosyltransferase domain of Large has properties of a UGGT and the second of a typical glycosyltransferase. Co-transfection of Large mutants affected in the different glycosyltransferase domains did not lead to complementation. While Large mutants were more localized to the endoplasmic reticulum than wild-type Large or revertants, all mutants were in the Golgi, and only very low levels of Golgi-localized Large were necessary to generate functional alpha-DG. When Large was overexpressed in ldlD.Lec1 mutant Chinese hamster ovary (CHO) cells which synthesize few, if any, mucin O-GalNAc glycans and no complex N-glycans, functional alpha-DG was produced, presumably by modifying O-mannose glycans. To investigate mucin O-GalNAc glycans as substrates of Large, a new CHO mutant Lec15.Lec1 that lacked O-mannose and complex N-glycans was isolated and characterized. Following transfection with Large, Lec15.Lec1 cells also generated functionally glycosylated alpha-DG. Thus, Large may act on the O-mannose, complex N-glycans and mucin O-GalNAc glycans of alpha-DG.
Figures


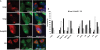
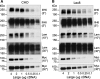

 ); GlcNAc (▪); Man (
); GlcNAc (▪); Man ( ).
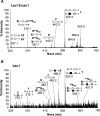
).
References
-
- Arnold SM, Fessler LI, Fessler JH, Kaufman RJ. Two homologues encoding human UDP-glucose:glycoprotein glucosyltransferase differ in mRNA expression and enzymatic activity. Biochemistry. 2000;39:2149–2163. - PubMed
-
- Arnold SM, Kaufman RJ. The noncatalytic portion of human UDP-glucose: Glycoprotein glucosyltransferase I confers UDP-glucose binding and transferase function to the catalytic domain. J Biol Chem. 2003;278:43320–43328. - PubMed
-
- Barresi R, Campbell KP. Dystroglycan: From biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. - PubMed
-
- Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Zhang W, Schachter H, Dumanski JP, et al. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med. 2004;10:696–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

