Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD
- PMID: 19470693
- PMCID: PMC2713471
- DOI: 10.1182/blood-2009-03-208983
Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD
Abstract
Historically, graft-versus-host disease (GVHD) beyond 100 days after hematopoietic cell transplantation (HCT) was called chronic GVHD, even if the clinical manifestations were indistinguishable from acute GVHD. In 2005, the National Institutes of Health (NIH) sponsored a consensus conference that proposed new criteria for diagnosis and classification of chronic GVHD for clinical trials. According to the consensus criteria, clinical manifestations rather than time after transplantation should be used in clinical trials to distinguish chronic GVHD from late acute GVHD, which includes persistent, recurrent, or late-onset acute GVHD. We evaluated major outcomes according to the presence or absence of NIH criteria for chronic GVHD in a retrospective study of 740 patients diagnosed with historically defined chronic GVHD after allogeneic HCT between 1994 and 2000. The presence or absence of NIH criteria for chronic GVHD showed no statistically significant association with survival, risks of nonrelapse mortality or recurrent malignancy, or duration of systemic treatment. Antecedent late acute GVHD was associated with an increased risk of nonrelapse mortality and prolonged treatment among patients with NIH chronic GVHD. Our results support the consensus recommendation that, with appropriate stratification, clinical trials can include patients with late acute GVHD as well as those with NIH chronic GVHD.
Figures
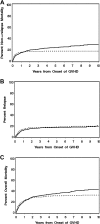
 ). In all 3 panels, progression to NIH chronic GVHD was treated as a competing risk for patients who presented initially with late acute GVHD.
). In all 3 panels, progression to NIH chronic GVHD was treated as a competing risk for patients who presented initially with late acute GVHD.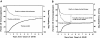
 ). (B) The cumulative incidence of discontinued systemic treatment among patients who presented initially with late acute GVHD (—), together with the competing risks of death or recurrent malignancy during systemic treatment with (
). (B) The cumulative incidence of discontinued systemic treatment among patients who presented initially with late acute GVHD (—), together with the competing risks of death or recurrent malignancy during systemic treatment with ( ) or without (- - -) the additional competing risk of transition to NIH chronic GVHD. Areas in each panel are labeled according to the initial event after starting treatment. The “Therapy continuing” areas include only patients without a competing event.
) or without (- - -) the additional competing risk of transition to NIH chronic GVHD. Areas in each panel are labeled according to the initial event after starting treatment. The “Therapy continuing” areas include only patients without a competing event.
References
-
- Lee SJ, Vogelsang G, Flowers MED. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. - PubMed
-
- Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man: a long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. - PubMed
-
- Sullivan KM. Graft-versus-host disease. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. Malden, MA: Blackwell Sciences; 1999. pp. 515–536.
-
- Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. - PubMed
-
- Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104:3501–3506. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

