Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity
- PMID: 19470704
- PMCID: PMC2736088
- DOI: 10.1210/en.2009-0221
Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity
Abstract
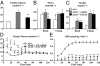
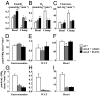
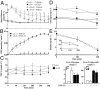
Fibroblast growth factor 21 (FGF21) is a novel metabolic regulator shown to improve glycemic control. However, the molecular and functional mechanisms underlying FGF21-mediated improvements in glycemic control are not completely understood. We examined FGF21 effects on insulin sensitivity and glucose fluxes upon chronic (daily injection for 8 d) and acute (6 h infusion) administration in ob/+ and ob/ob mice. Results show that chronic FGF21 ameliorated fasting hyperglycemia in ob/ob mice via increased glucose disposal and improved hepatic insulin sensitivity. Acute FGF21 suppressed hepatic glucose production, increased liver glycogen, lowered glucagon, and improved glucose clearance in ob/+ mice. These effects were blunted in ob/ob mice. Neither chronic nor acute FGF21 altered skeletal muscle or adipose tissue glucose uptake in either genotype. In conclusion, FGF21 has potent glycemic effects caused by hepatic changes in glucose flux and improved insulin sensitivity. Thus, these studies define mechanisms underlying anti-hyperglycemic actions of FGF21 and support its therapeutic potential.
Figures
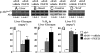

References
-
- Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M 2008 FGF21 attenuates lipolysis in human adipocytes: a possible link to improved insulin sensitivity. FEBS Lett 582:1725–1730 - PubMed
-
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E 2007 Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5:426–437 - PubMed
-
- Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A 2008 FGF21 corrects obesity in mice. Endocrinology 12:6018–6027 - PubMed
-
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA 2007 Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425 - PubMed
-
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB 2005 FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

